The 2016 Nobel Prize in Chemistry is awarded to Jean-Pierre Sauvage, James Fraser and Bernard Feringa for their development of molecular machines thousands of times the size of a human hair. This is the story of their success in connecting molecules together to create molecular machines such as a tiny elevator, motors and even artificial muscles.
How much can a running machine be minimized? This is the question asked by the Nobel laureate Richard Feynman, who received much publicity thanks to his prediction from the 1984s about the development of the field of nanotechnology, a question raised in his revolutionary lecture in XNUMX. Barefoot, wearing a pink polo shirt and beige pants, he turned To the audience and asked: "Now we will talk about the possibility of manufacturing machines that include moving parts that will be extremely tiny."
He was sure that machines could be built on the scale of nanometers. Such machines already exist in nature. He gave the example of the bacterium's whips, corkscrew-like molecules whose seven turns allow the bacterium to move forward. However, could humans - with the help of their large hands - build machines so small that you would need a microscope to see them?
Future vision - molecular machines will arrive in 30-25 years
One possible way would be to build a pair of mechanical hands that would be smaller than our hands, which in turn would allow the construction of a pair of even smaller hands, and so on. This possibility has been explored, but without much success, says Feynman.
Another strategy, in which Feynman believes more, would be to build the machine from the bottom up. In this theoretical idea of his, different materials, such as silicon, would be sprayed onto a surface, one layer of atoms after another. After that, some of the layers will partially melt and be removed, creating moving parts that can be controlled with an electric current. According to Feynman's vision, such an array could be used to create an optical aperture for a tiny camera.
The purpose of the lecture was to inspire researchers from among the audience, and allow them to examine the limits of what is possible. When Feynman folded his papers at the end of his lecture, he looked at the audience and said, in a playful tone: "Have a wonderful study in designing various types of familiar machines, we'll see if you succeed. And invest in it for 30-25 years, then they will have a practical use. What it will be, I don't know." What neither Feynman nor any of the researchers in the audience knew at the time, is that the first step towards the development of molecular machines had already been taken, but in a slightly different way than Feynman had envisioned.
Molecules are combined as machines
In the mid-twentieth century, as part of an effort to develop increasingly advanced molecules, chemists attempted to produce molecular chains that included ring molecules linked together. The researcher who succeeds in this will not only create a new and amazing molecule, but also a new type of chemical bond. Normally, molecules are held together by strong covalent bonds where the atoms share shared electrons. The vision was to create instead mechanical bonds, where the molecules are combined together without the atoms reacting directly with each other (Figure 1).
In the 1983s and XNUMXs, several research groups reported that their test tubes contained molecular chains, but the quantities they synthesized were small and the methods were so complex that their use was extremely limited. Advances in this field were at the time considered more of a purely scientific curiosity than applied chemistry. After years of various obstacles, many researchers raised their hands in this field and in the early eighties the entire field was almost completely eroded. However, the important breakthrough came in XNUMX. Using a common copper ion, a research group from France, led by Jean Pierre Sauvage (Jean-Pierre Sauvage), managed to take control of the molecule.
Jean-Pierre Sauvage succeeds in binding molecules around a copper ion
As often happens in research, the inspiration came from a completely different area. Jean-Pierre Sauvage worked in the field of photochemistry, where chemists develop molecular conjugates capable of capturing the energy stored in the sun's rays and using it to drive chemical reactions. When Jean-Pierre Sauvage built a model of one of these active photochemical couplings, he suddenly saw the structure's resemblance to a molecular chain: two molecules intertwined around a copper ion.
This insight led to a dramatic change in Jean-Pierre Sauvage's research fields. Using photochemical coupling as a model, his research group synthesized one ring-shaped molecule and a second crescent-shaped molecule so that both were attracted to the copper ion (Figure 1); The copper ion provided a kind of adhesive force that held the molecules together. In a second step, the group used a chemical substance in order to fuse together the saharon molecule with a third molecule to form a new ring, and in the process create the first link in the chain. In the next step the researchers removed the copper ion which served its purpose.
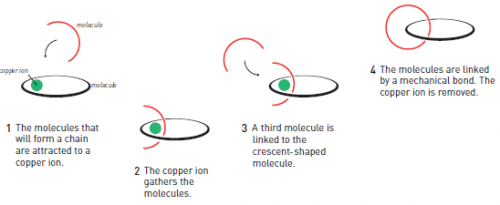
Chemists refer to the utilization of the reaction: the percentage of reacting molecules that give rise to the target molecule, the product. In the previous attempts to create molecules in the form of chains, the researchers achieved, at best, a few percent of survival. Thanks to the copper ion, Savage managed to increase the survival to an impressive value of 42%. Suddenly, molecular chains were more than just a matter of curiosity.
With his revolutionary method, Savage revitalized the field of Topological chemistry in which researchers - who usually use metal ions - combine different molecules together in increasingly complex structures, from long chains to complex clusters. Jean-Pierre Sauvage and James Fraser Stoddart are leaders in this field and their research groups have created molecular versions of cultural symbols such as the trefoil knot, Solomon's knot, and Boromine rings (Figure 2).
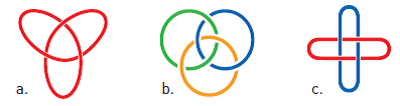
The first steps to the development of a molecular motor
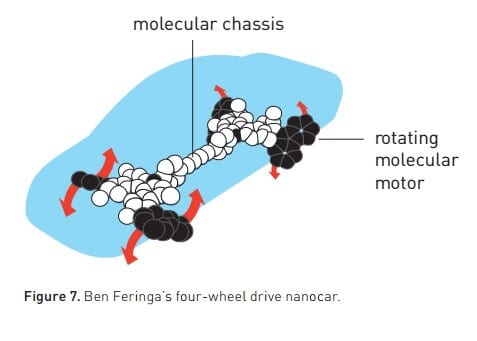
Jean-Pierre-Sauvage soon realized that molecular chains (Shashman Little ones, catenanes, from the Latin word for chain, chain) not only constitute a new family of molecules, but it also started the first step towards the creation of molecular machines. In order for a machine to perform its tasks, it must contain several parts that can move. The two combined rings fulfill this requirement. In 1994, Jean-Pierre-Sauvage's research group also succeeded in synthesizing a catanane in which one ring looped, in a controlled manner, around the other ring after energy was added to the system. This molecule was the first embryo of non-biological molecular machines.
The second embryo of molecular machines was created by a chemist who grew up on a farm in Scotland that had no electricity or modern conveniences.
Fraser Stoddart threads a molecular ring inside a molecular rod
As a child, Fraser Stoddart did not watch television or use a computer. Instead, he occupied himself with building aggregates, a talent that could certainly help a chemist: knowing shapes and understanding how they can fit together. He was drawn to chemistry also out of his desire to become a molecular artist - sculpting new forms, ones that humanity has never seen before.
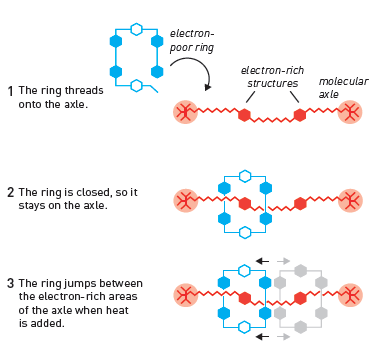
When Fraser Stoddart developed one of his molecular creations that forms the basis of the 2016 Nobel Prize in Chemistry, he also took advantage of chemistry's ability to design molecules that attract each other. In 1991, his research group synthesized an electron-deficient open ring, as well as a long rod, or axle, with electron-rich structures at two sites (Figure 3). When the two molecules met in solution, the electron-deficient structure was attracted to the electron-rich structure, and thus the rod was threaded into the ring. In the next step, the research group closed the opening in the ring and thus it remained threaded into the rod. In this way, the researchers synthesized structures from the rotaxane family (rotaxane): a ring-shaped molecule mechanically attached to the axis.
In the next step, Fraser Stoddart took advantage of the freedom of movement of the ring in order to drive along the axis. When the solution is heated, the ring moves back and forth - similar to a tiny elevator - between the two electron-rich parts of the spindle (Figure 3). In 1994, he managed to achieve complete control of this movement, thereby eliminating the randomness that in other cases controls movements within chemical systems.

8 תגובות
Gentlemen, long before nanobots in our bloodstream it will be possible to simply replace the damaged organ with a structure produced by XNUMXD printing. Just this week we saw in Israel the replacement of a vertebra in the spine, which was created by XNUMXD printing after scanning the affected area of the patient.
In my opinion, the nanometer machines will have much more far-reaching applications, not only in medicine but also in artificial intelligence, quantum computers, microscopic neurons, efficient photovoltaic cells (even energy production in artificial photosynthesis that will be more efficient than the chlorophyll molecule), espionage, etc.
As far as the patents are concerned - there are a million "holes" in every patent, what's more the patent offices are not stupid and will know how to deal with a flood of nanometer machines.
A few things: First, there can be a problem if group A has several patents and group B has a collection of other and complementary patents. Both groups can interfere with each other and block them from reaching a complete and implementable technological solution. Something that may significantly delay the development of the technology until some legal (or technological) solution is found.
Second, I wouldn't underestimate the FDA and CE approvals to be obtained. The technologies are getting more and more sophisticated. Their approval takes a very long time and the discovery of malfunctions and recalls of defective medical products is something completely uncommon. You should think several times before allowing robots that may not have been properly tested to enter the body and make changes in it. It would not be very pleasant to find out that the robots, inspired by some software glitch, turned you into a schnitzel.
rival
Let's start from the end - yes, we have several patents. The value of a company also depends on the number of patents it has.
The approval process (by the FDA, that's what I know) only gets longer over time. More conditions are constantly being added, to give better "undercover" to the approving bodies. A possible way to shorten the process is a process where you show that you are similar to an existing product that already has approval. This is how we work, but it limits us because it is difficult to introduce revolutions (and there are some...).
Regarding the surgical process, the situation is complicated, but in principle you cannot sue a surgeon for patent infringement, even though there are patents for such processes... read here:
http://www.patenteducation.com/images/200902_Limited_Monopoly_-_Patenting_Surgical_Procedures.pdf
Miracles,
This is true for today, but in my opinion the approval times for new drugs will get shorter and shorter, for example we will have quite accurate simulations of different body organs, kidneys, liver, brain... it will be possible to test the effect of drugs in a much faster way, it will also be possible to deliver drugs directly to the affected organ and test the their effect without affecting other body parts.
Regarding the patents, I'm not really familiar with it, but I'm sure that even today there are many treatment methods that are the unique invention of a scientist or research groups, are these methods protected by a patent and not allow every hospital and country to use them? Are the imaging methods used in your work protected by a patent? Are you the only ones allowed to use them?
rival
It sometimes takes 10 years to approve a new drug. Approving something completely new may take even longer.
And there is another problem - as soon as something starts to be economical, the patents will start. Imagine someone succeeding in getting a patent for "inserting a machine into a living body that attacks harmful cells". It happens in every other field, so why not here?
Miracles,
Also in my opinion this is more or less the timetable in which these things will happen, only I am a little more optimistic than you and in my opinion the lower end of your scale is the more realistic. I see at what pace things develop and it is not linear.
rival
I'm betting on 30-50 years.
So much news about breakthroughs in the field of nanotechnology, I wonder how soon we will see Ray Kurzweil's nano robots circulating in our bloodstream repairing and strengthening our bodies from the inside.