Physicists from universities in Sweden and Finland have discovered an efficient way to synthesize graphene nanoribbons directly inside single-walled carbon nanotubes
Physicists from universities in Sweden and Finland have discovered an efficient way to synthesize graphene nanoribbons directly inside single-walled carbon nanotubes.
The findings were recently published in the scientific journal Nano Letters.
The material graphene, a monoatomic layer of clean carbon, has a wide range of unusual and particularly fascinating properties. As an electrical conductor, this material performs its activity as well as copper. As a heat conductor, it performs its activity better than all other known materials. Considerable changes in the properties of graphene can be achieved by preparing it in the form of strips of variable thickness, known as nanostrips. These nanostrips are now a practical focus of interest in the fields of physics as a particularly promising material in the development of electronic components, solar cells and many other products. However, making these nanoribbons has never been easy.
A joint team of physicists from Sweden and Finland led by Professor Alexandr Talyzin has discovered a way to use the empty spaces inside carbon nanotubes as a one-dimensional chemical reactor to make encapsulated graphene. A fascinating feature of this space is that different chemical reactions take place within it compared to three-dimensional conditions in a normal medium.
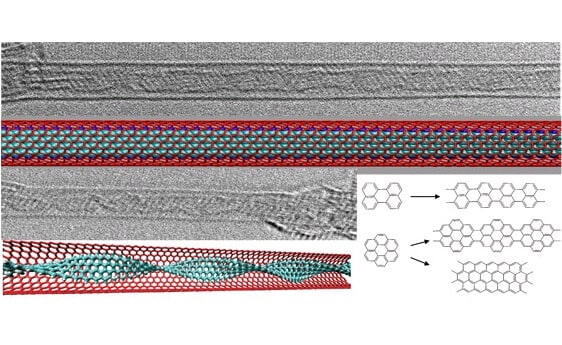
"We used the materials coronene and perylene, which are long organic molecules, as building blocks to prepare narrow and elongated graphene nanostrips inside the tubes. The idea of using these molecules as building blocks for graphene synthesis was based on our previous research," notes the lead researcher.
In our research we discovered that the coronene molecules are able to react with each other under certain conditions to form dimers, trimers and longer structures in their powder form. The findings indicated the possibility that the coronene molecules could be used to prepare graphene, but it was necessary to stabilize them on a common plane in order for the required reaction to occur. The inner space of single-walled carbon nanotubes seems to be an ideal place to force the molecules to arrange themselves in the "end-to-end" shape so necessary for the polymerization reaction.
In the new study, the scientists showed that this is indeed possible to do. When the researchers looked at the first samples through an electron microscope, they made an exciting discovery: all the nanotubes were filled with graphene nanoribbons. "The success of the experiments also depended to a large extent on the selection of the particular nanotubes. Nanotubes of suitable diameter and high quality were provided to us by our colleagues at Aalto University," says the lead researcher.
In the next step, the researchers found that the shape of the graphene nanoribbons trapped in the tubes can be changed through the use of different types of aromatic hydrocarbons. The properties of the nanostrips are very different and depend on their shape and thickness. For example, the nanoribbons can be metallic or semiconducting depending on their thickness and type. It is interesting to note that the nanotubes themselves can also be metallic, semi-conducting or insulating depending on their diameter.
“This capability leads to enormous potential for the development of a wide variety of applications. In the future, we will be able to prepare hybrid materials that combine graphene and nanotubes in all possible combinations," explains the researcher.
For example, metallic nanostrips inside electrically insulating nanotubes form ultra-tiny insulated wires. It will be possible to use them directly inside nanotubes in order to produce light, so that we get nanobulbs. Semiconducting nanostrips could be used for the development of transistors or solar cells and a combination of metallic-metallic structures could form a completely new type of nanocable - an electrical conductor that could be used, for example, in transmitting radio signals.
The new method for synthesizing hybrid materials is simple, easily scalable, and allows a filling level of the tubes in the nanostrips of almost 100%. Theoretical simulations also showed that the graphene nanostrips retain their unique properties inside the nanotubes since they are protected from their external environment.
"The new material looks particularly promising, but we still have to conduct extensive research in the fields of physics and chemistry related to it. The synthesis of the material is only the beginning. Now we are interested in studying its electrical, magnetic and chemical properties and understanding how hybrid materials can be used in practical applications," explains the lead researcher.
The news about the study

One response
What is meant by the sentence "Nano strips can be metallic.." Does it mean that they will have electrical conductivity like metal or does it mean that they will be "contaminated" with metal? (like the silicon in semiconductors).