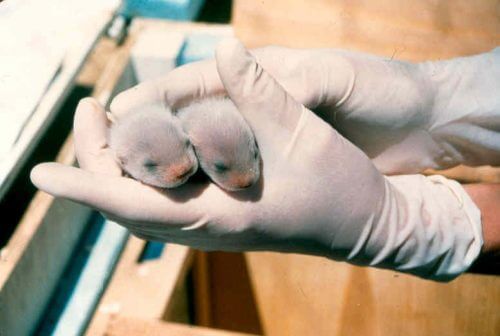
Efforts to save the black-footed ferret in the US in the hope of increasing the genetic diversity of the species by reintroducing ancient DNA into the population

As far as we know in 1987 there were only 18 left Black-footed ferrets in the world But thanks to captive breeding and intensive management of the population, they already number several hundred today. However, like many other species that have recovered after their numbers have declined so much, all individuals today are actually half-siblings: almost genetic clones. Everyone is therefore prone to the same hereditary health problems and those disease-causing and environmental changes that could lead to the elimination of the population. In an effort to increase the genetic diversity of ferrets and their chances of survival in the long term, at the US Fish and Wildlife Service (Fws) are considering an extreme idea: a plan to re-introduce DNA that has been lost in the contemporary population but is still found in individuals that have died a long time ago and are stored in zoos and museums. The effort may not sound as strange as the dream of reviving the woolly mammoth, but it certainly requires reviving dead genes with their hosts - no easy task.
The black-footed ferret's genetic bottleneck was even worse than it sounded. Of the 18 ferrets the FWS rescued 30 years ago from the American West, only seven passed their genes on to future generations. "Every black-footed ferret alive now is descended from seven individuals," says Kimberly Fraser, spokeswoman for the FWS Black-footed Ferret Conservation Center. "Take seven people, what will you get?"
In 2015, hydrogen The project for the genetic rescue of endangered and extinct species של The fund for long-term thinking The determination of the genetic sequence of two living ferrets and the DNA sequence of a male and a female that died in the 80s and are kept frozen inConservation Research Center At the San Diego Zoo. A comparison between the pairs shows genetic variation in the stored ferrets that can be returned to the living population through cloning or gene editing technology CRISPR. The method has been studied in attempts to revive species such as the passenger pigeon, and it can theoretically create live clones of the frozen ferrets that can then drown the surviving ferrets. Alternatively, the cloned genomes can be used to integrate into the DNA of the surviving ferrets DNA sequences that include the code for antibodies against two common infectious diseases: A type of rabies וa bitch. Another option is to remove the genes that make ferrets susceptible to these diseases from their DNA. "To add two more heads of genetic lineage? It's a lot," she says Ryan Phelan, CEO of the Genetic Rescue Project.
The ferret has several advantages in such a project: ferrets reproduce quickly and have relatives that thrive in the wild and can be used as surrogates in the initial cloning study. But it is not surprising that this type of genetic editing faces challenges such as finding adequate funding and overcoming legal obstacles that lurk in genetic projects involving endangered species. And besides that, there are also the scientific hurdles that include the arduous effort to create a viable clone and the long-term decision-making process as to which genes to add and which to subtract. The Genetic Rescue Project is preparing to begin the initial gene editing work in cell culture in 2016 under the auspices of the San Diego Zoological Society, assuming funding and researchers are found.
If genetic rescue can help the black-footed ferret thrive, it may also be effective in other animals and plants that conservationists are trying to save. Like amphibians that were almost wiped out from the world because of the disease it causes The chytrid fungus and the so-called pocket animal Tasmanian devil whose population is infected with contagious cancer of the face. In fact, a similar genetic conservation effort is already underway to save the northern white rhinoceros, a subspecies of which only three individuals remain. In this attempt, frozen sperm from dead male rhinos and "artificial gametes" will be used: stem cells that have been selected through genetic engineering and made sex cells that contain versions of restored genes. The international team of scientists carrying out the mission recently published its plan In the article In the online journal Zoo Biology in which he wrote that "[the northern white rhinoceros] can be considered doomed to extinction, unless extraordinary efforts are made to prevent this fate."
In all these cases there are also ethical considerations. For example, an important argument against trying to reverse extinction is that money should not be wasted on reviving the mammoth as long as there are real elephants in need of saving and that the limited funding sources would be better directed to protecting habitats or supporting infrastructure that combats poaching. In any case, the ferret project will show that the methods for reversing extinction are applicable methods, which can also be used for the real conservation of existing species that are on the verge of extinction. As Shaplan claims: "The question is: at what point should we as managers help populations that do not have the evolutionary adaptability that they could have if the state of affairs were different?"
The goal the Fish and Wildlife Service is aiming for is a wild population of 3,000 fertile ferrets scattered in 30 different populations, and finally to rehabilitate ferrets in the last place they were found in the wild: Mittites in the state of Wyoming. But without the genetic restoration efforts, the reproduction of the remnants could deteriorate their numbers or even lead to their extinction. "I don't think it's possible to invest 100 years in a captive breeding program with these genetic limitations," says Fraser, a spokeswoman for the Fish and Wildlife Service. "I hope [the genetic rescue] happens before I die."

One response
It's such a shame that the translators keep making mistakes in interesting lists,
It says: "…… genetics involving endangered species…….."
Will the translators fail to learn and internalize that
There are no "varieties" in zoology?
There are families, genera, species (and even subspecies),
But "varieties" yuk!