What is the connection between bubbles in a carbonated drink, clouds and crystals? The physics and chemistry of agglomeration - a daily subject whose theory behind it is not simple.

Beer, like all carbonated drinks, is saturated with molecules Carbon dioxide (CO2) dissolved, that is, the CO molecules2 surrounded by water molecules that do not allow them to stick to each other. Above the liquid, however, the CO2 It is in a "normal" gaseous state at a pressure of about 2 atmospheres (about the same as the pressure in the car's wheels). Every fraction of a second some of the CO molecules2 that in a gas pass into a liquid, while some molecules are released from the liquid into a gas. If we don't shake the bottle for a while, the transition rates will equalize, and the so-called state will be obtained 'Dynamic equilibrium'.
What happens when you open the cork? The compressed gas in the nozzle area is released out at once, thus throwing the system out of equilibrium. To reach a state of equilibrium again, CO molecules need2 Many are released from the liquid into the air, but since they cannot be released immediately, they do so slowly and patiently in a way called 'bubbles'. Note that in order to create a separation between the gas package (bubble) and the liquid, energy must be invested. why? We can think of two groups of people (say, blues and reds) mixed with each other uniformly. If the movement of people is random, there is very little chance that homogeneous groups of people of one type, say, reds, will form. In thermodynamic terms, in order for this to occur degree of disorder (entropy) should decrease, so it is not a process that occurs spontaneously.
Another reason is a reason related to the chemical bonds - the bubble needs to move the water molecules away from each other, for this the relatively strong bonds between the water molecules (hydrogen bonds) must be broken. Both reasons emphasize that the release of gas from liquid is not a simple process.
Given that a relatively high investment of energy is required, which is not normally available, how do bubbles form anyway? Recall the groups of people mixed together, and let's assume that in some places there are announcers who call out on megaphones "All the reds to me!" It is conceivable that around the announcers, 'red' groups will form and grow. The physical equivalent of the announcements is called 'nucleation sites' and the clustering process is callednuclearization': Tiny particles in the liquid form nuclei around which gas molecules cluster in order to create the initial surface that separates them from the liquid. From the moment a tiny (microscopic) bubble is formed, much less energy is needed and therefore the process unfolds by itself until the formation of a large (macroscopic) bubble that floats to the top. Other sites that serve as bubble nucleation sites are air bubbles that are there to begin with - for example in small cracks in a glass. The existing bubble already creates an area where there is no water - expanding an existing bubble requires much less energy than creating a new bubble.
By the way, there are many processes in nature, in order to start them, a relatively high initial energy is necessary - an energy threshold called activation energy. These processes would not take place if it were not for those 'intermediaries' whose action parallels the action of the nucleation sites. For example, countless biological processes are activated by enzymes which are large molecules on the back of which biochemical reactions can occur relatively easily, such as The ribosomes of Ada Yonat who rose to greatness. Although, the same processes would have occurred without the mediation of the enzymes, but it would have taken much longer. In chemistry the same mediator is called 'catalyst' (catalyst). There are reactions that are enough to provide the activation energy at the beginning, similar to the spark that starts the combustion or as Bruce Springsteen sang: You can't start a fire without a spark Then the reaction itself provides the energy. But there are reactions that require a continuous supply of energy.
Back to the capital. As stated earlier, nucleation sites can also be microscopic cracks in the glass. The next time you look at a glass of carbonated drink, remember that the bubbles are not randomly formed everywhere in the liquid. In fact, there are chains of bubbles that can be traced back to specific points in the glass: these points are microcracks that serve as nucleation sites. When a carbonated drink is poured into a glass, a lot of foam is created because all the microscopic cracks act as serial bubble generators: every time a bubble is released, the site that created it vacates to create another bubble and so on and on until the CO runs out2 Or that a new dynamic equilibrium is reached. To avoid the foam, bartenders know that the inside of the glass must be wetted first, but why? The water molecules temporarily 'block' the macroscopic defects in the glass and thus significantly reduce the chance of bubbles forming. The difference is impressive - almost no foam is formed.
'Mentos in Coke' He is one of the experiments that became a cultural icon thanks to hundreds of videos uploaded to YouTube by any 11-year-old with a video camera. At first it wasn't clear to me why a huge geyser sprang from the bottle's mouth - it seemed almost unnatural. Is there a mysterious substance in Mentos that makes the gases release powerfully? It turns out not. Although the mantis candy looks and feels smooth, in fact, macroscopically, it is extremely rough. As it sinks into the drink, the many pores on it form nucleation sites for the CO2 dissolved in coke and at once countless bubbles are formed that are released with jet power and sweep away some of the liquid with them. The full explanation more complex (And there is also in Wikipedia), but that's the basic idea. The team of the fascinating program Time Warp, who specializes in fast photography (many frames per second) examined the issue:
As evidence that there is nothing special about Mentos, a similar effect is obtained even if we sprinkle sand, sugar or salt on our carbonated drink as you can see HERE.
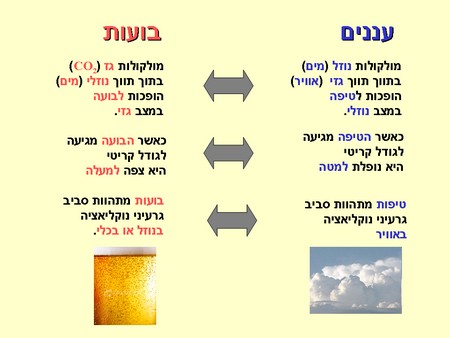
the book Clouds in a glass of beer Describes simple experiments that illustrate physical principles of weather phenomena. In the first chapter The author explains why in many ways, clouds They are the mirror image of bubbles in a carbonated drink. Clouds form when the relative humidity high enough for water molecules in a gaseous state (steam) to condense into small droplets. In order to hasten the rain, sprinkle in the air (or actually, 'sow') tiny particles that will form nucleation nuclei for the droplets, and this is analogous to the dispersion of salt in beer which accelerates the formation of bubbles. You can, of course, add more details to the inverted parallel:
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
Before we say goodbye to the beer, let's examine another interesting phenomenon. When you open the cap, a tiny cloud appears at the top of the bottle. What is this cloud and how was it formed? First, let's recall a well-known thermodynamic principle: a rapid change in gas pressure entails a change in temperature. For example, when a gas is compressed quickly its temperature rises (a bicycle pump heats up during inflation) and when a gas expands quickly its temperature drops (a compressed gas tank cools down after a few clicks). By the way, we encounter this principle every time we useAir-Conditioner or in a refrigerator based on cycles of compression and volume increase of gas. In light of this, it is possible to understand why the drop in pressure at the nozzle of the bottle lowers the temperature there. Believe it or not, but it is a drastic drop to around minus thirty degrees Celsius, which causes the water vapor that is there to immediately condense into tiny water droplets, that is, to become a cloud (or fog). If there were nucleation nuclei in the nozzle area, such as tiny dust particles, the same condensation would also be obtained at normal temperatures, as is demonstrated This simple experiment which you can make at home, if the beer is not available in the meantime It occurred to you.

8 תגובות
Hi, this is Oren Farber (the writer)
My new blog is here: orenmada.net/blog
With lots of new content…
I ask that no one do any experiments with father's beer.
Dad needs her for some peace of mind.
sympathetic,
Why not try this at home?
Unless you meant that children should not drink beer - with that I completely agree. They can, however, carry out experiments on father's beer!
a student
In my opinion just from watching the video there is more here than a large surface area to create marble bubbles
Lamentos. It seems to me that the short scale of time is because there is a phenomenon here that amplifies itself.
I will try to explain. The mantus is a nucleation center but it is not enough that they were so
In fact the typical time scale was the time it takes to gas in this case C02
flow into the mantus I assume because he would have done so by diffusion and the time scale was
Relatively slow.
In my opinion, the mantus is a nucleation center and carries gas to it. The flow of gas to the mantus increases
the creation of the bubbles, while the bubbles increase the flow of the gas to the Mentos. There is a phenomenon here
increasing itself. Bubbles create flow to Mentos and flow to Mentos creates more bubbles.
In such a situation I expect that there will be a certain threshold for the phenomenon, or a typical size for Mentos capable of producing such a flow.
What will happen if we throw half a Mentos or a quarter? Will the flow decrease by 2 times or 4 times I don't think so
Because it is an exponential growth.
Kids don't try this at home!
Ehud, the subject is probably more complex than the simplistic explanation in the article. In order to understand such a phenomenon accurately, a full statistical thermodynamic treatment should be done with a combination of surface chemistry.
In general, the surface area of the mentos is relatively large (it is rough and not smooth, therefore there are many dents and fissures in the surface, which greatly increases the surface area), and there are many times a much larger number of nucleation sites than in a glass. That's why when you insert the Mentos, you take the system out of control and the rate of nucleation increases very quickly, and you get the "boom".
Let's assume theoretically that there was an infinite amount of dissolved CO2 in the glass, after a certain time the nucleation around that Mentos candy would have reached 2 and the rate of foam formation would have reached a maximum. If at this stage you had put in another candy, you would have doubled the number of nucleation sites and the "amount" of foam would have doubled (assuming that the foam formation is linear to the number of nucleation sites). This is of course a very general and imprecise description, but that's the idea.
It seems that the phenomenon is much more complex than described in the article.
Questions that arise are:
What determines the typical time scale? in a glass of formation beer
The bubble is slow in the case of the mantos and gives the impression that the rate of occurrence
It is exponentially faster. What causes the different behavior?
Does Mentos have an exponential number of nucleation centers or is it
In a phenomenon that increases itself?
That's why I love science
How many times have you looked at the bubbles that form in a glass of beer or soda and asked yourself how they are formed?
I wouldn't mind taking a physics and chemistry lesson over a glass of beer.