An Israeli innovation to fight hard-to-heal wounds: the FDA approved Anziserge to market a broad-spectrum bactericidal solution that is resistant to other antibiotic drugs, as well as fungal infections
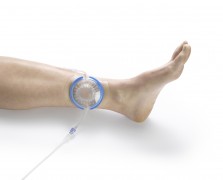
EnzySurge, a provider of innovative solutions for the advanced treatment of chronic wounds, announced today that it has received approval from the American Food and Drug Administration (FDA) to market the SilverStream™ solution, intended for the treatment of difficult-to-heal wounds, such as ulcers, pressure sores, diabetic ulcers and wounds after surgery.
Enzisurge is the developer of the DermaStream®, a system for treating wounds through the continuous and controlled flow of active healing solutions, such as the SilverStream, while creating negative pressure over the wound. The combined treatment of the device and the solution removes foreign substances, microorganisms and unwanted waste from the wound. The solution can be used by instillation directly into the wound, or by using the DermaStream device.
According to Dr. Noa Hadar and Prof. Amichai Freeman, who led the development, the unique composition of the SilverStream solution, which contains, among other things, low concentration silver ions, has been proven in the laboratory to kill bacteria in a wide range of resistance to other antibiotic drugs, as well as fungal infections. At the same time as the approval of the SilverStream, the FDA also approved a version of the product intended for use "over the counter" (for sale without a prescription in pharmacies), which will be marketed under the name DermaSept - this version is intended for the treatment of minor cuts, burns and skin irritations.
"We are happy to receive FDA approval for the unique product we developed," says Amir Shiner, CEO of Anziserge. "We believe that SilverStream is a powerful and innovative solution for wound care and that it will be a very significant addition to DermaStream. The combination of the two will become in the coming years the natural choice for anyone looking for a comprehensive system for treating wounds. The product will be launched on the market in 2010 through our partner in the USA - Virginia Biosciences Commercialization Center".
The treatment of diabetic wounds, pressure sores and various ulcers is expensive and complex and requires continuous supervision and treatment by professionals. The global market for chronic wound care products was approximately $3.2 billion in 2008 and its volume is expected to grow to approximately $4.1 billion in 2011.
"Anziserge's recent achievements, and especially the FDA approval, represent a significant opportunity for us to make a mark in the global wound care market in the near future," says Scheiner.
Enzisurge Ltd. develops and provides innovative solutions for the treatment and management of chronic wounds. The company's flagship product - the DermaStream offers a unique and innovative treatment method developed by the company, which includes the continuous flow of active therapeutic solutions over the controlled wound environment. DermaStream - which includes a device and a solution - enables comprehensive, effective and comprehensive treatment throughout all stages of chronic wound treatment. The company's product line is intended for use in hospitals, clinics and the patient's home.

4 תגובות
If this is true, it is a revolution, where can it be purchased in Israel?
The key word is low concentration in the charlatans there is no uniform concentration, and usually toxic amounts as well.
My father, will his skin turn black?
I went to their website - what is the antiseptic ingredient?
May silver solution!!!
It's a loop, isn't it?
To buy enziserg or not to buy enziserg