Researchers from Eindhoven University (TU/e) have developed a completely new method for initiating chemical reactions. For the first time they were able to use mechanical forces to control catalytic activity - one of the most important activities in chemistry and biology. This discovery opens a window for the development of materials capable of repairing themselves following the effect of mechanical stress.
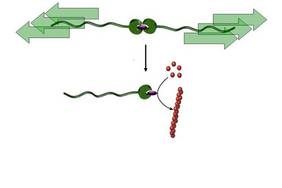
The research team, part of the Institute for Complex Molecular Systems (ICMS) and part of the Department of Chemical Engineering, both at the University of Eindhoven, is the first to demonstrate that a "dormant" catalyst can be activated by pulling a polymer chain, a "molecular pull rope". The researchers were able to use this catalyst to initiate a variety of chemical reactions, including polymerizations - the creation of polymer chains from smaller molecular building blocks called monomers.
This discovery opens the way to creating self-repairing materials that become stronger in response to mechanical stress. For example - if a material breaks, at the same time the metallic coupling splits into two halves while activating the catalyst that causes the immediate repair of the material.
This discovery could also lead researchers to other applications where it will be possible to turn on and off chemical reactions at will. Possible applications include precision injection molding to create plastic products, where the new method could simplify the process, or in chemical syntheses on a tiny scale.
How does it work? the weakest link:
The researchers fully packed a catalytically active metal ion between two molecular caps (ligands). They tied two polymer chains to these covers creating a long chain with a metal clasp in the center. These conjugates were dissolved in a liquid that had undergone ultrasonic "irradiation" to create bubbles in the liquid. When these bubbles collapsed, they produced an extremely strong gravitational force that stretched the chains and eventually resulted in the breaking of the "weak link" - the metal coupling - in two.
The cover on one side of the array was now open, the active metal ion was released and thus became active as a catalyst. That is - now it can be active in accelerating various chemical reactions.
The research findings were published in the new journal Nature Chemistry.
