Chemists from the University of California Los Angeles and the University of Washington have succeeded in creating custom-designed enzymes. If this becomes commercial, it will be possible to use them to produce energy, to clean the environment from polluting substances and countless other actions
Chemists from the University of California Los Angeles and the University of Washington have succeeded in creating custom-designed enzymes. This technology, when perfected, could change the face of the world. The research was published in the prestigious scientific journal Nature on March 19.
Enzymes are nature's efficient little machines. They exist in their millions inside every cell, every bacterium and every virus. They produce energy from nutrients and sunlight with high efficiency, move cells with the help of tiny propellers or whips and are able to break down - and assemble - almost any organic material.
Since the idea of nanotechnology was raised, enzymes have become the gateway to a new and extraordinary world. If we can design new enzymes, then we will have extraordinary control over all the processes that occur in nature. The enzymes will be able to imitate any natural process in an efficient, green and energy-efficient way. They will be able to break down molecules of air pollution into carbon, oxygen and hydrogen. They will be able to pray from salt water and sewage water. They will even be able to photosynthesize and generate electrical energy from sunlight. Enzymes that perform these actions already exist in nature, but the protein designers will be able to produce them more efficiently, so that they will combine with other enzymes that can provide them with energy and in this way small, efficient and multi-part machines will be created. The next step will be designing and creating enzymes capable of performing processes that cells do not normally bother to perform. An example of such a process is taking carbon atoms and assembling them into a flawless diamond. Since carbon is one of the most common materials on earth, it is possible that diamond will be the cheapest and strongest building material in the future.
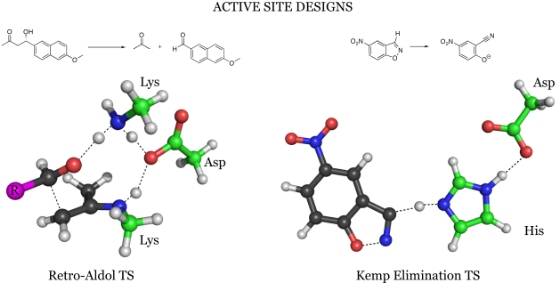
Now two groups, in cooperation, have succeeded in designing and creating an enzyme that leads to a chemical reaction, which according to the researchers does not exist in nature. This reaction is called Kemp elimination and during it a hydrogen atom is separated from a carbon atom. In a previous study published in the journal Science, the chemists in the group were able to design and create another synthetic enzyme that catalyzes a retro-aldol reaction, during which a bond between two carbons is broken.
Professor Kendall Hauck's research group includes 30 computational chemists who use supercomputers to study chemical reactions based on insights from quantum mechanics, and to arrive at a mechanism by which the enzymes can reproduce them. The researchers designed the active site of the enzymes - the area where the chemical reactions take place - by investigating possible combinations of chemical groups, and determining which ones are most suitable for producing the desired chemical change. They then determined the exact three-dimensional arrangement of these chemical groups within the active site, with a resolution of less than a hundredth of a nanometer. Drawings of the active site were given to a group at the University of Washington led by biochemist David Baker. Baker and his group used computer programs to design the amino acid sequence that would become the enzyme's active site, then actually created the enzyme.
The future is already here?How far are we still from the vision of nanotechnology, of enzymes working at the service of man?
Hauk does not believe that we will reach this technology in the coming years. He emphasizes the difficulty in designing new enzymes in a period of days or weeks, compared to the perfection that nature has selected and developed over billions of years of evolution. His colleagues in the profession, on the other hand, remain optimistic. Fernando Clement, one of the authors of the article in Science, says that, "Most scientists thought it would be impossible, and we also felt that way after many failures. But improvements in planning and sophistication led to success in the end."
Whether it will take a few years, or whether it is a slow revolution of decades, there is no doubt that the breakthrough will be remembered in the future. The two research groups succeeded for the first time in creating and designing man-made enzymes, thus opening the door wide to a better, greener and healthier future.
Image :

17 תגובות
Dance,
There is a world of difference between liposomal membranes with mitochondria and several enzymes, and whole cells.
In my opinion, artificial cells will be more efficient than normal cells (in the very limited sense of performing the task), since it is likely that in our artificial cells we will not need most of the cellular mechanisms. For example, the cell skeleton, the cell division mechanism and a large part of the mechanism for creating proteins. Normal cells consume a lot of energy to control these mechanisms.
We don't want to create normal cells that can perform a thousand and one functions, but specialized cells that can only do one function and then die. Such a role for example would be breaking down molecules of air pollution.
I want to return you to your original claim:
"Enzymatic reactions will never be able to break down molecules of air pollution into carbon, oxygen and hydrogen to precipitate salt water and wastewater or take carbon atoms and assemble them into a flawless diamond."
From what you have written in recent messages, you agree that enzymatic reactions can carry out the processes listed above under the right conditions, as they do in cells. The problem, if I understand you correctly, is simply that the conditions in which the enzymes will work (inside cells) were not mentioned in the article. It seems to me that in the discussion held between us now, we also dealt with this issue in detail - and resolved it.
Roy
Unfortunately, liposomal membranes will not be sufficient for enzymatic catalysis of endothermic processes. You also need mechanisms that will provide energy to the aforementioned enzymes, i.e. mitochondria, and then we are getting very close to whole cells. Which raises the question of why not use cells in advance (fungal bacteria and the like).
Dance,
I apologize. Apparently I should have explicitly mentioned that the article refers to enzymes that are inside membranes. I don't know of enzymes that work in air without an aqueous (or fatty) environment around them, so I didn't see the need to point out that we can't just release enzymes into the air.
At the same time, the creation of simple liposomal membranes is a technology that has been known for decades, as well as liposomes within liposomes. I don't see a fundamental problem in adding enzymes of the right type to the membrane, so that we can create selective partitions that will pass the raw materials between them.
Roy
The processes you mentioned do happen in cells and given similar conditions, the same ones you mentioned will definitely happen elsewhere. But to remind you, the reactions mentioned in the article do not occur in cells and all reactions in cells occur in an aqueous medium. Therefore, either very complex systems will be required to eliminate the products of the enzymatic reactions or complex systems for transferring the reactants and products to an aqueous or at least liquid phase (enzymes can work in organic solvents). The creation of the enzymatic groups is not the problem, but the creation of the membranes that will separate the products of the enzymatic reactions from the enzymes.
Dance,
If I understand your argument correctly, you are saying that energetically stable molecules will not be able to be broken down (or assembled) by enzymes into less stable (more energetic) molecules. You set the temperature, the concentration of the reactants / products, etc. as a condition for the formation of a reaction.
However, the fact is that this happens in cells in an excellent way.
Part of the point is that cells have a modulation of processes. A certain enzyme turns raw materials into products, then the products are removed from its working environment (with the help of another enzyme), to allow the concentration of the reactants to become high again.
I don't see why it wouldn't be possible to do something similar with well-designed groups of enzymes working cooperatively.
To Roy
I didn't claim that enzymes don't work, the opposite is true, enzymes work their action is blessed and the utilization of its energy is phenomenal, and there are still things that enzymes are unable to do.
Dance,
In this case, how would you explain how the enzymes in cells turn small organic molecules into complex polymers? Alternatively, how do they turn large organic molecules into much smaller organic molecules, from which other molecules are then composed?
All these processes require energy obtained from ATP. The point is that the cell also has very efficient mechanisms to create the energy. This is not a leading perpetum, but simply mechanisms with a very high utilization of energy.
Leroy, A. Thank you for taking the trouble to translate the interesting article and other articles that you and the site team translate.
And to your question I will explain my argument. Enzymes (and any other catalyst) all their power is summed up in that they lower the energy barrier of chemical reactions. They do not change the equilibrium concentrations of the chemical products. Therefore, molecules made of carbon and hydrogen oxygen, since they are more stable (are at a lower energy level) than the free elements will be stable even in the presence of the enzymes that are exactly suitable for their discharge reaction. This is true for any normal temperature, at very high temperatures the equilibrium tends towards the discharge of the aforementioned molecules, but at this temperature the enzymes are not stable.
And in the words of a physicist, if there is an enzyme that transfers compounds to carbon, oxygen and hydrogen without investing a lot of energy (high temperature) then you can build a leading perpetuum where on one side the compounds will be formed in an exothermic reaction where you can use the heat generated in the reaction to create electricity for example, and on the other side you will break down the The reaction products without a large energy investment.
In contrast, pollutants that are oxygen-nitrogen compounds can (theoretically) be broken down with the help of catalysts or enzymes
Just to dance,
Could you explain why you think enzymatic reactions could never, for example, break down air pollution molecules into carbon, oxygen and hydrogen?
A basic understanding of the essence of the enzymes will lead to the conclusions that the authors of the article were sailing on the wings of their imagination and therefore enzymatic reactions will never be able to break down molecules of air pollution into carbon, oxygen and hydrogen to precipitate salt water and leachate or take carbon atoms and assemble them into a flawless diamond.
Some scientific accuracy is desirable on a scientific website.
I especially laughed at the line: "Diamond may be the cheapest and strongest building material in the future."
Imagine all the women with diamond jewelry that cost hundreds of millions, who will suddenly realize that they are walking around with rings and earrings embedded in the modern equivalent of concrete! As the saying goes: "Grace is vanity and beauty is a lie..."
I wonder if it can be used in the very distant future, I can only speculate, for artificial panspermia. Sending a spaceship that will only contain fuel for long space flights and probiotic soup with enzymes programmed to accelerate evolutionary processes, you can also add some viruses and bacteria for the probability that some will survive and develop.
One day we will reach another world and discover there a race we created. Just don't start thinking that we are their gods and worship us as believers. 😉
What's new,
Each case individually, of course. But at least in the case of solar cells, I don't know of a method that can achieve better utilization of sunlight than the molecular machines that participate in photosynthesis.
Regarding the production of hydrogen, I don't think the goal of the researchers was to produce hydrogen with the help of Kemp elimination. If I'm not mistaken, Kemp's elimination is used as part of molecular synthesis processes.
The question is what is the usefulness of using enzymes to validate other processes, for example:
1. Production of hydrogen by electrolysis or another process
2. Solar cells
Would it be possible to obtain a higher efficiency than the processes mentioned by using artificial enzymes fused in bacteria?
Eyal,
They say that - "from the day the Temple was destroyed, prophecy was taken from the prophets and given to fools and infants."
I prefer not to predict and declare myself a fool, but I must admit that I am personally very happy to see the success of this research, and see it as a breakthrough that can greatly advance the field of nanotechnology.
Sounds very interesting. It seems that a window has been opened for a new and complete industry in size, as it will be said in 10 years - enzyme engineering. And if the business is recognized under the title "nano-technology" then that will become its main point. What do you say Cezana? 😉