A recent discovery of the chemical conversion of the greenhouse gas carbon dioxide into its useful form paves the way for scientists to discover or engineer an organism that can perform this very task.
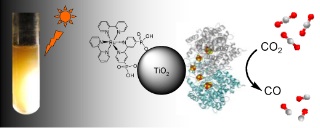
Biochemist Steve Ragsdale from the University of Michigan and a team of other researchers from the University of Oxford in the UK have found a way to efficiently convert carbon dioxide into carbon monoxide using sunlight. The research findings were published in the scientific journal Journal of the American Chemical Society.
Not only is this a demonstration that a common compound can be converted into another commercially useful compound using lower energy inputs than existing methods, it is also a method that is not so different from the processes that organisms in nature carry out every day.
"This is the first step in demonstrating the possibility of the existence of this process, and imagine bacteria that will do it for us," adds the researcher. "I don't know of an organism that uses the energy of sunlight to activate carbon dioxide and turn it back into carbon monoxide, but I suppose we could find one, or we could genetically engineer it."
The research team was able, through the use of an enzyme-based titanium oxide, to cause the electrons from the carbon dioxide to become energetically excited and jump to an enzyme that then catalyzes the redox reaction to the carbon monoxide form. A photosensitizer connected to titanium allows the use of visible light to perform the process. The enzyme present in the system is much more active than other catalysts, allowing the conversion to be carried out repeatedly. Its unique advantage - it is able to get very close to the oxygen atom.
"We were looking for a way similar to the one that exists in nature, but even more effective. The advantage is that we will be able to produce a chemical system with these properties on a commercial scale," notes the lead researcher.
The product of the process - carbon monoxide - is a desirable chemical that can be used in other chemical processes to create electricity or hydrogen. Carbon monoxide also has an important value as a fuel and can be converted by known catalysts into hydrocarbons or methanol used as liquid fuel. Although carbon monoxide is used as a source of energy and as biomass for bacteria, it is toxic to humans and therefore it is necessary to find ways to handle and use it safely during chemical reactions.

7 תגובות
I hope someone will refer to such an old article...
Not sure I understood something...
Energy is needed to recycle the fuel and produce fuel from it, since we produce energy and use it to produce energy. Of course, as in any process, there is a loss of energy along the way, so why do it in the first place?
And if you say that this is how we reduce GHG emissions into the atmosphere, then you can simply stop producing polluting energy and consume energy directly from the sun/wind, etc...
Can someone enlighten me?
Max:
The combination of the two comments above yours explains the logic.
CO is a toxic gas that must be avoided in the environment of animals (including humans).
However - there is no obstacle to producing it in a protected and controlled manner in an environment where it will only be used as fuel and its toxicity will not be manifested.
to my people,
Probably more stable polymers can be made, and the carbon monoxide can be used as a better initiator. there is
Such a possibility when carbon monoxide is heated to a high boiling point and it becomes a radical.
I wonder what energetic processes can be obtained from carbon monoxide. Maybe in the formation of aldehydes? Does anyone know and can tell?
I don't understand they installed a catalytic converter in vehicles to turn carbon monoxide into carbon dioxide and now they are going to turn carbon dioxide into carbon monoxide does anyone understand the logic
Guru:
Your memory does not deceive you, but the article refers to the matter (in the last sentence).
Forgive me for trying my memory here, but isn't carbon monoxide a poisonous gas that prevents the red blood cells from carrying oxygen? I think I learned something like that in elementary school