Without collagen we would fall apart - in the full sense of the word. This large protein molecule, the most common in our body, is the "glue" that sticks the tissues.
Without collagen we would fall apart - in the full sense of the word. This large protein molecule, the most common in our body, is the "glue" that sticks the tissues. In fact, in our distant evolutionary past it was collagen that allowed our single-celled ancestors to evolve into multicellular creatures - because it helped individual cells stick together.
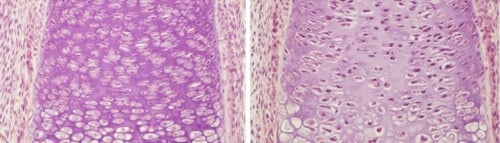
During the development of the fetus, large amounts of collagen are formed in it, the starting material for building the bone. Since the production of collagen is a complex and multi-step process, which requires a lot of oxygen, it could be expected that the growing bone would receive a large supply of oxygen. But surprisingly, just the opposite is true. The bone, as an internal organ, receives small amounts of oxygen - in relation to the external tissues of the fetus. Furthermore, the cartilage tissue, which precedes bone formation, actively pushes out the blood vessels that carry the oxygen.
A study conducted at the Weizmann Institute of Science and published in the journal Development sheds new light on the paradox. Although the riddle concerning bone formation in the presence of so little oxygen has not yet been solved, the research findings explain how the collagen is nevertheless produced under conditions that are apparently so unsuitable.
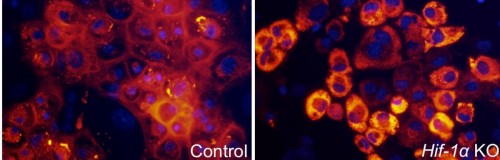
Prof. Elazar Seltzer, Little Ben Tovim, and the research student at the time Dr. Roi Amriglio, from the Department of Molecular Genetics, studied the formation of collagen in the growing bone of mouse embryos. They focused on a molecule called HIF1-α, which plays a central role in controlling the cell's response to low tissue oxygen levels. The scientists discovered that HIF1-α also functions as a central control switch in the building of collagen, and makes the process extremely efficient, in order to optimally utilize the little oxygen. When the scientists blocked the activity of this molecule, collagen production was impaired, and the bone did not grow properly.
The scientists found that HIF1-α acts like a competent manager: it creates optimal conditions for building collagen. First, it increases the creation of the catalytic enzymes that accelerate the process. In addition, it stops other metabolic processes in the tissue, so that the little available oxygen is directed entirely to the cells that produce the collagen, with the aim of allowing them to concentrate on this task only.
These findings clarify how the most central building blocks are formed in the fetal body. In addition, the research may contribute to the understanding of diseases. For example, it may offer an explanation for the question, why low levels of oxygen in cancerous tissues do not prevent tumor development.
connected

Bones, muscles and tendons give our body its shape, maintain its stability, and allow it to move. But in order to perform these functions successfully, they must form a single system with highly coordinated parts - the skeletal and muscular system. A new study conducted at the Weizmann Institute of Science and published in the journal Development discovered a central mechanism responsible for the creation of the system.
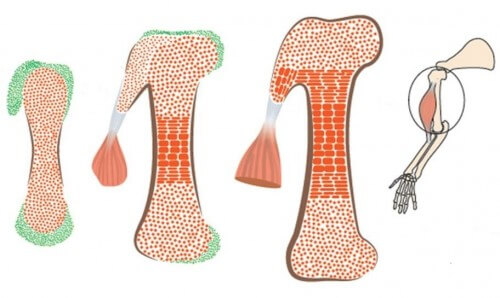
A basic step in the assembly of the skeletal and muscular system is the development of the connection units between the bone and the tendons. These are bumps of different shapes and sizes, growing on the surface of the bone. These protrusions are essential for the proper functioning of the skeletal and muscular system: they form stable grip points for the muscles, which attach to the bone via the tendons, and disperse the tension exerted by the contracting muscles on the bone.
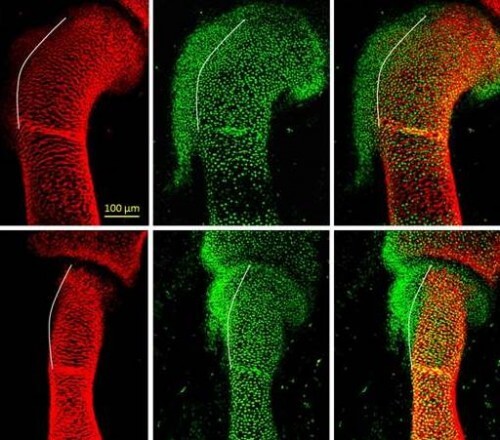
In the new study, which was done in mouse embryos, the scientists discovered that the bone protrusions are formed by a different group of cells from the known bone-building cells, which was not known before. The cells that build the protrusions are characterized by a kind of "split personality": they are controlled at the same time by two genetic programs - one suitable for bone, and another suitable for tendons. This splitting is what allows the connection of the tendons and muscles to the bony protrusions.
The scientists deciphered the details of the two genetic programs, including their molecular control mechanisms, by creating mutant mouse embryos, in which certain genes were missing, and monitoring their development. The research was carried out by Prof. Elazar Seltzer and the research student at the time, Dr. Einat Blitz, from the department of molecular genetics at the institute.
The discovery that the bones are formed in the fetus in a modular way may help to explain their mechanical properties, for example, the ability of different anatomical areas of the bone to handle stress and weight in different ways, which contributes to the resistance and flexibility of the skeleton.

One response
References Please