The pharmaceutical company GlaxoSmithKline (GSK), yesterday launched a new vaccine to prevent cervical cancer, Cervix, intended for the first time for girls and women from 10 to 45 years of age.
The pharmaceutical company GlaxoSmithKline (GSK) launched yesterday (November 4.11.2008, 10) at a press conference, a new vaccine to prevent cervical cancer intended for the first time for girls and women from the age of 45 to XNUMX. The vaccine, called Cervarix, was recently approved by the Ministry of Health and will be offered to girls and women in Israel as part of The supplementary insurances of Maccabi Health Services, United Health Insurance Fund and Leumi Health Insurance Fund.
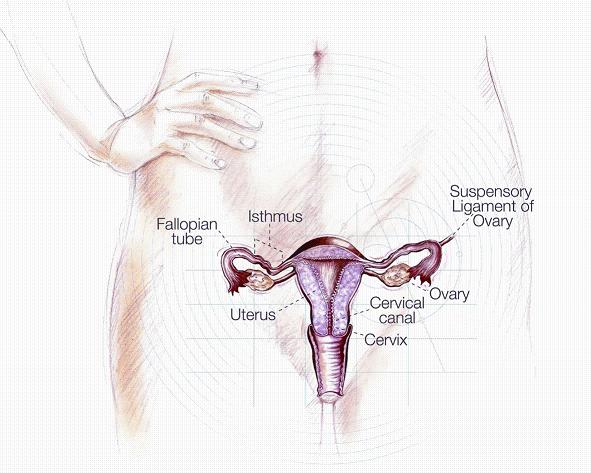
The new vaccine is designed to protect against different strains of the human papillomavirus, which is responsible for about 80% of all cervical cancer cases and is given in three doses of an injection in the arm over a period of six months, with the second injection being given about a month after the first injection and the third injection being given about six months after the injection the first The virus, which is usually transmitted during sexual intercourse, is carried by the man and may affect the cells in the tissue of the cervix and lead to the development of persistent infections, warts and pre-cancerous changes. Following this change process, cancer may develop. Vaccinating girls and women against the virus is an effective and safe preventive tool against cervical cancer, which may save their lives. Cervarix, is intended for vaccinating girls and women between the ages of 10 and 45, compared to the vaccine currently available on the adapted market
Today for women up to the age of 26 only.
Assistive system
Cervarix is the first vaccine to prevent cervical cancer, which includes in its composition, a unique adjuvant system called AS04. This system increases and intensifies the immune response of the woman's body to the antigens contained in the vaccine and thus extends the duration of protection.
Preventive treatment
In large-scale clinical studies, which included over 40 women, Cervix demonstrated 100% safety and high efficiency in the prevention of cervical cancer, which is caused, as mentioned, by the papilloma virus of strains 16 and 18, which are responsible for 70% of cervical cancer cases. In addition, it was found that the vaccine also protects against infections caused by other strains of the virus types 45, 31 and 52 which together are responsible for another 12% of cervical cancer cases.
The longest lasting immune protection against cervical cancer
At the annual conference of the Society of Gynecology Oncology, which was recently held in Florida, the results of a six-and-a-half-year follow-up study were presented, in which 776 women aged 15 to 25, from South America and Brazil, who received the Cervarix vaccine, participated. An optimistic picture was obtained from the follow-up results, from which it appears that among 98% of the women who were vaccinated with Cervix, high levels of antibodies against the papilloma virus were maintained, which are 11 times the level of antibodies produced in the body due to natural infection, caused by the virus of strains 16 and 18. So in fact, for at least six and a half years from the date of vaccination, a high efficiency of 100% has been proven, in preventing pre-cancerous conditions and cervical cancer. Moreover, the study showed a high efficacy of 78% in preventing cervical cancer caused by the virus strain 45 and 60% efficacy against the virus strain 31 over the same period of six and a half years, which are the third and fourth causes of cervical cancer. As mentioned, this is the longest period, during which high levels of antibodies against the papillomavirus strains 16 and 18 were maintained as a result of the cervical cancer vaccine, which was reported until
Today in the world of science.
Effective in preventing persistent infections and the development of pre-cancerous cells
Any sexually active woman may be infected with the papilloma virus and the risk of persistent infection increases with age as a result of the virus. Thus, according to global assessment data, approximately 80% of women will be infected with the papillomavirus during their lifetime, with approximately 50% of these infections being caused by viruses from the strains that can cause cervical cancer. According to evaluation data from the Israeli Society for Colposcopy and Cervical Pathology, approximately 1,300 women in Israel are diagnosed each year with pre-cancerous changes in the cervix, which may lead to cervical cancer. Cerverix has demonstrated a high efficiency of 100% in preventing the development of pre-cancerous cells caused by the virus from the strains 16 and 18 and as having an efficiency of 72% in preventing the development of pre-cancerous cells caused by all types of the virus. Because of this, the vaccine is a step that may reduce mental stress, serious illness, mortality and save economic resources for the Israeli economy.
Data on cervical cancer in Israel and around the world
According to the data of the Cancer Society, about 180 women are diagnosed every year in Israel with cervical cancer, most of them as a result of the human papilloma virus. According to global assessment data, cervical cancer is the second most common malignant disease among women under the age of 45, in developed countries, with approximately 500 women diagnosed with cervical cancer every year, a number that is expected to grow every year and even double by 2050 If they do not take preventive measures such as vaccination. Every year about 270 thousand women die from cervical cancer and it is the third leading cause of death in the world, after breast cancer and lung cancer.
Confirmation of vaccination
The Cervarix vaccine, recently approved by the Ministry of Health, previously received the approval of the European Medicines Agency (EMEA) as well as the approval of the Australian Medicines Agency (TGA) (Therapeutic Goods Administration of Australia) and is approved in 80 countries around the world. These days the vaccine is offered to girls and women in Israel as part of the supplementary insurance of Maccabi Health Services, the United Health Insurance Fund and the National Health Insurance Fund.
Research among the public on behalf of the Geocartography Institute
The Geocartography Institute conducted a study among women aged 18-45 in order to learn about the habits of women visiting the gynecologist, performing a cervical smear, the sexual behavior of the interviewees, examining the knowledge women have about the papilloma virus, their willingness in principle to receive the vaccine and/or purchase it for Their daughters and barriers to receiving the vaccine. The survey was carried out using a computerized telephone survey system, in a sample of 512 women aged 18-45 years, among whom 15% are mothers of girls/girls aged 9-20. The statistical error range for this sample is +4.33%, at a statistical confidence level of 95%. The interviews were conducted from the computerized call center at the Geocartography Institute in early June 2008.
The research findings show that over half (54%) of the women aged 18-45 visit a gynecologist for a periodic check-up, at least once a year, with women aged 25-45 attending at higher rates than those aged 18-24. Half of the years they performed an examination of the surface of the cervix, where the rate of operations is twice as high among women aged 2-25 than among women aged 45-18.
It is known that women's sexual behavior such as starting intercourse at a young age is a risk factor for infection with the papilloma virus, which causes cervical cancer. The average age of starting intercourse in this study is 18.8 years, with 10% starting intercourse at the age of 15-16 and another third at the age of 17-18. Others report that they started at an older age. Multiple spouses is also a risk factor. In the survey women stated that they had sex with 3.1 partners on average before the first wedding and in total they had sex with 3.7 partners on average.
What do women know about the papilloma virus?
In total, about 40% were able to state that the virus could lead to the development of cervical cancer, at the same time another 40% had not heard of the papilloma virus and another 17% could not state details, so there is more to teach the public. The women's sources of information about cervical cancer are mainly the gynecologist (42%) and then the Internet.
Do women want to get vaccinated?
The information received shows that more than half of the women (55%) indicate that they will buy the vaccine, including 2% who have already been vaccinated, and a similar proportion of women (53%) will buy the vaccine for their daughters. For now, 30% of women indicate that they think or are sure that they will not be vaccinated, but only 15% indicated this regarding their daughters' vaccination. The main reason why women do not get vaccinated, which was stated in the highest rates (20%) is "not relevant to my age", other reasons were stated in lower rates, but now that there will be a vaccine against cervical cancer that can be given to women up to the age of 45, the situation will change. We asked the women at what age women should be vaccinated against cervical cancer, and almost two-thirds of the women (63%) indicated age 25 or older. Now with the new vaccine that allows women over the age of 26 to be vaccinated, it will be possible to meet the needs of most women.

6 תגובות
It's hard for me to know who to believe
I found the following article successful and balanced
Dr. Harper, who helped develop the vaccine at the vaccine company Mirak, talks about risk versus benefit
and makes order in Balgan
http://www.cbsnews.com/stories/2009/08/19/cbsnews_investigates/main5253431.shtml
Raya, what is written on the Emet Else website should be taken with a little salt and a lot of pepper. These are delusional people who are not ready to accept rational explanations but only conspiracy theories, some of which don't even fit together well. As for the HPV virus and cervical cancer, a year ago the virus and the connection to cervical cancer was discovered Nobel Prize for Medicine. But who are the members of the Nobel Prize committee against some delusional conspirators.
I've heard some weird conspiracy theories about this vaccine.
For example, on this site:
http://www.emetaheret.org.il/2008/12/27/%D7%96%D7%94%D7%99%D7%A8%D7%95%D7%AA-%D7%97%D7%99%D7%A1%D7%95%D7%9F-%D7%A6%D7%95%D7%95%D7%90%D7%A8-%D7%94%D7%A8%D7%97%D7%9D-%D7%95%D7%99%D7%A8%D7%95%D7%A1-%D7%94%D7%A4%D7%A4%D7%99%D7%9C%D7%95%D7%9E-2/
They say there:
The HPV Scam Revealed:
"During the last few years, the HPV vaccine was sold to the public, and to many girls who were forced to take it, based on the claim that it prevents cervical cancer.
The FDA has been aware for several years that there is no connection between the HPV virus and cervical cancer.
This is true?!
Is there a connection between HPV and cervical cancer?
Sarsarix is a vaccine that has many side effects. Read here:
http://vaccinesinc.blogspot.com/2010/02/blog-post_23.html
I just have a question: is the vaccine effective even after the woman has already been exposed to the virus? (That is, does the vaccine work by increasing the immune response to the virus, even if the virus is already present in the woman's body).