A research team from Illinois has managed to overcome one of the main obstacles to the development of a promising technology that will both reduce the emission of greenhouse gases into the atmosphere and help in the production of fuel
A research team from Illinois has managed to overcome one of the main obstacles to the development of a promising technology that will both reduce greenhouse gas emissions into the atmosphere and help fuel production.
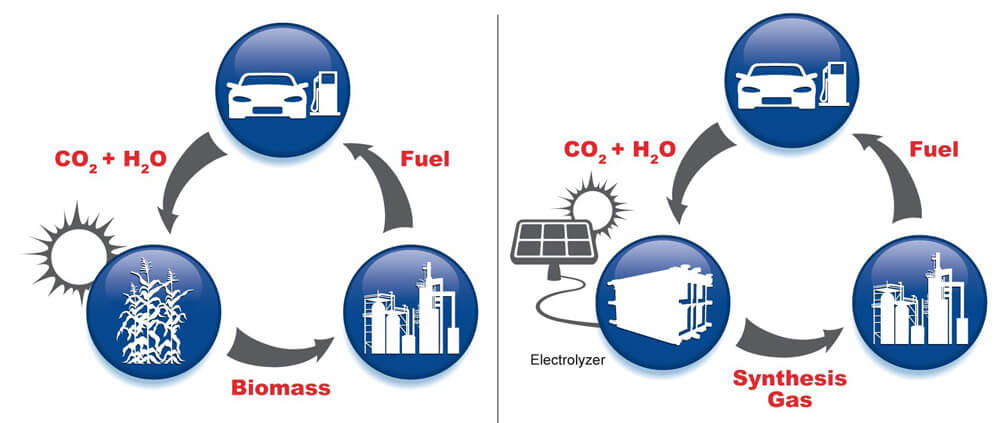
University of Illinois chemical and biological engineering professor Paul Kenis and his research team are trying to develop a catalyst to improve the process of artificial photosynthesis. Artificial photosynthesis is the process by which the gas carbon dioxide is converted into useful carbon-based chemicals, the most prominent of which are fuel or other compounds derived from petroleum.
In plants, the process of photosynthesis uses the sun's energy to convert carbon dioxide and water into sugars and other carbohydrates. Biofuels are obtained from sugars extracted from crop plants such as corn. However, in artificial photosynthesis, an electrochemical cell uses energy coming from a solar collector or a wind turbine to convert the gas into simple carbon fuels, such as formic acid or methanol, which in turn are then processed to obtain ethanol or other fuels.
"The main advantage is that the process is not competitive with food supply," says the lead researcher, "and it is much cheaper than transporting biomass by ship to the refinery." However, one of the biggest setbacks of this process is the first stage where the carbon dioxide gas is converted to carbon monoxide gas, the stage that requires a large amount of energy. This stage consumes such a large amount of energy that a situation is reached where more energy is used to produce the fuel than the final amount stored in it.
The research group from Illinois turned to another, innovative approach, which utilizes an ionic liquid in order to accelerate the reaction, thus significantly reducing the amount of energy required to initiate the reaction. The ionic liquids stabilize the intermediate forms obtained during the reaction so that a smaller amount of energy is required to complete the reaction.
The researchers used an electrochemical cell in a reaction device that allows separation between the amount of incoming carbon dioxide and the amount of oxygen emitted in the reaction. The design of the cell allows the researchers to adjust the composition of the electrolyte flow in order to improve the reaction kinetics, including the addition of ionic liquids as a catalyst. "This catalyst significantly reduces the potential required to convert carbon dioxide, and thus it is possible to use a smaller amount of energy."
Next, the researchers hope to tackle the yield problem. In order to make their technology have commercial applications, the researchers need to increase the speed of the reaction and maximize its utilization.

8 תגובות
If I understood correctly - then the researchers proved that the process works and now it needs to be made commercial. That is, moving from the research phase to the engineering phase. I hope that there will indeed be someone who will dare and finance this engineering phase. Even if we were not in a situation of global warming, there are two problems with the use of fossil fuel - one is the limited amount of fuel which causes its price to rise and the wealth of various dictators who for some reason control the production of oil in the world. This problem, in my understanding, can be solved using the process mentioned in the article. The second problem - the pollution of the air and the environment resulting from the fuel - will probably not be solved even if the process in the article becomes commercial, since polluting carbon fuel will still be used. .
You're right, Yotzemach, it's because the fuel companies prefer to invest in lobbying the Senate and the Knesset and prevent all proposals designed to encourage the use of such substitutes.
And the local authorities have also become greedy
http://www.nrg.co.il/online/1/ART2/312/602.html?hp=1&cat=459&loc=3
Morning news I read articles about one or another development in the subjects of alternative fuel sources, green energy and so on, some days I finish reading and go to the nearest gas station to fill up 35 liters of ancient fossil remains.
light:
Indeed, I did not notice this sentence in the article (and I did not notice that you wrote the sentence in brackets).
Indeed this sentence is trivial and probably its entire purpose is an advertisement for the current discovery, which probably also uses more energy than it stores.
The real innovation is only in efficiency, but there is no new quality advantage of the kind that this sentence implies.
Michael
I did not write this, it is written in the article and noted as "one of the biggest failures of this process". It is clear to me that the process requires more solar energy than is stored in the fuel, so this is not the major drawback of the process.
light:
With a name like yours you should have realized that even though what you said was true it was completely irrelevant.
Why? And what does it have to do with your name?
Because the energy used is the energy of light (well, to be more precise - solar energy in the form of light or wind) which, if they had not captured a part of it with the method in question, would have been lost (at best) or contributed to the warming of the earth (at worst).
"More energy is used to produce the fuel than the final amount stored in it." - Obliged by the Energy Conservation Law
Fuel is fuel is fuel even in the plural!
"D.L.K.M" .... Yuk!