In recent years, researchers have discovered that water flows spontaneously inside extremely tiny tubes of graphite, graphene, called carbon nanotubes
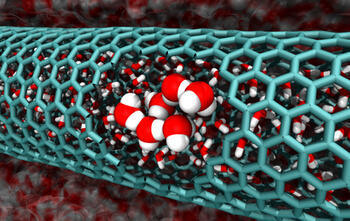
Scientists sometimes reveal strange and unexpected findings when they look at materials at their nanometer level - the level of individual atoms and molecules. This is true even for the most common substances, such as water.
In recent years, researchers have discovered that water flows spontaneously inside extremely tiny tubes of graphite, graphene, called carbon nanotubes. This unexpected observation is fascinating because carbon nanotubes hold promise in the emerging fields of nanoflow and nanofiltration, where nanotubes may help maintain tiny flows or separate impurities from water. However, no one has been able to explain how, at the molecular level, a stable liquid tends to confine itself to such a small domain.
Now, using an innovative method by which the dynamics of water molecules can be calculated, researchers from Caltech believe they have solved the mystery. It turns out that entropy, the measure of disorder, was the missing factor.
"This is quite a surprising result," says William Goddard, professor of chemistry, materials science and applied physics at Caltech. "Researchers usually focus in this field on energy and not on entropy." This is because water forms an extensive network of hydrogen bonds, which makes them extremely stable. Breaking these close relationships requires an investment of energy. And since enough bonds must be broken in order for the water to flow in small nanotubes, it is unlikely that water will be made so easily.
"What we found is that it's actually bartering," notes the lead researcher. "You lose some of the good energetic stability that comes from the chemical bonds, but you gain in entropy." Entropy is one of the driving forces that will ultimately determine whether any process will occur by itself, or not. This measure represents the number of ways a system can exist in a certain state. The more possible arrangements a system has, the greater its disorder, and the higher the entropy. And in principle, nature tends towards disorder.
When water is optimally bound, all the hydrogen bonds "lock" the molecules in place, limiting their freedom and keeping the water entropy low. The researchers found that in the case of several nanotubes, water receives enough entropy by entering these tubes so that it exceeds the energy losses caused by the breaking of several hydrogen bonds. As a result, water flows independently into the tubes. The research findings were published in the scientific journal Proceedings of the National Academy of Sciences (PNAS).
The researchers examined carbon nanotubes with diameters ranging from 0.8 to 2.7 nanometers and found three reasons why water would flow freely inside the tubes, depending on their diameter. For the smallest nanotubes - between 0.8 and 1.0 nanometers - the tubes are so tiny that the water molecules line up in almost a single column inside them and are in a gas-like state. This means that the normal bonded structure of liquid water breaks down, giving the molecules much more freedom to move. This increase in entropy allows the water to enter the tube. For the next group of tubes - those whose diameter is between 1.1 and 1.2 nanometers, the confined water molecules organize into clumped, ice-like crystals. The researchers discovered that nanotubes of this type are the perfect size to "host" crystalline water. In this case the bonding relationships between the crystals, and not the entropy, are what allow the water to flow through the tubes.
For the group with the largest diameters, those spanning between 1.4 and 2.7 nanometers, the researchers found that the confined water molecules behave more like liquid water. However, again, some of the normal hydrogen bonds are broken, so that the molecules have more freedom of movement within the tubes. And the gain in entropy more than compensates for the loss in hydrogen bond energy.
Since the interior of the carbon nanotubes is too small for the researchers to examine experimentally, the team examined the dynamics of the confined water molecules using computer simulations. Using a new calculation method, they were able to calculate the entropy for the individual water molecules, while in the past such calculations were challenging and time-consuming - the old methods consumed 8 years of computer time in order to calculate the entropy that the researchers now calculate in only 36 hours.
The team also ran simulations using an alternative description of water – one in which water has the usual properties of energy, density and viscosity, but lacks the ability to hydrogen bond. In this case, the water did not tend to flow inside the nanotubes, a finding that provides further evidence that the extensive hydrogen bonds of ordinary water, which has a low entropy, lead to the spontaneous filling of the carbon nanotubes as the entropy increases.
The researchers believe that carbon nanotubes could be used in the design of supramolecules for water purification. Through the production of nozzles with the same diameter as the carbon nanotubes, it will be possible to develop a polymer whose purpose is to absorb water from a solution (of sea water, for example).

5 תגובות
man,
The formula that links "stability" and the tendency of a process to occur is expressed in the "Gibbs free energy" formula:
G = H − T*S.
G – Gibbs energy. In principle, the lower the Gibbs energy, the more likely the reaction is to occur and the more stable its final product (they tend to say that if the G of the product is lower than the G of the reactants, i.e. the G difference is negative, then the reaction is "spontaneous").
H - enthalpy energy, which mainly teaches about the degree of "energy stability" of the process. As you can see, the lower the energy, the more stable the material and the process.
T – temperature.
S – entropy (disorder), so it can be seen that the higher the entropy, the more the process and its final product will tend to occur.
A small note: this is a thermodynamic diversion that expresses how "stable" the process is, but does not directly teach about kinetics (how long it will take for the reaction to occur).
Of course, this is only a very comprehensive explanation. I can explain more but I think this is enough to answer your question.
Did someone say top perfume???
Reminds me of the infinite uncertainty engine from "The Hitchhiker's Guide to the Galaxy"
http://en.wikipedia.org/wiki/Technology_in_The_Hitchhiker's_Guide_to_the_Galaxy#Infinite_Improbability_Drive
man,
It's not a trade-off between entropy and energy, but a competition. A certain state of a system can be energetically more preferable, but at the same time entropically less preferable. The answer to the question of which of the two is more dominant will determine if the system will move to this state.
It is impossible to combine oranges with avocado, therefore there is no such amount. In closed systems the energy is (always) constant, and the entropy can change.
Thank you, father, very interesting.
If there is a trade-off between entropy and energy, is their sum (if you can call it that) in closed systems
will remain at a constant value?