The research findings significantly expand the scope of the use of metal-based catalysts that were discovered by the same researchers only three years ago
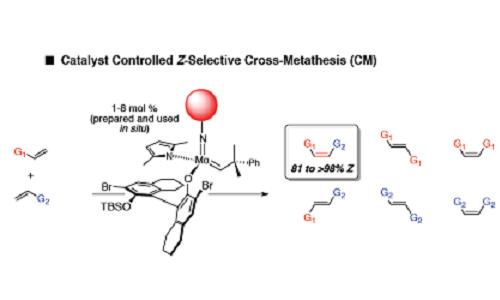
A new catalytic chemical method for the synthesis of an extensive and important set of carbon-carbon double bonds has been developed by scientists from Boston College and MIT universities. The research findings significantly expand the scope of the use of metal-based catalysts that were discovered by the same researchers only three years ago.
The catalysts, whose core is composed of the metal molybdenum, have now shown the ability to produce the higher energy isomer of an alkane molecule from two simpler and more readily available versions. The research findings were published in the prestigious scientific journal Science.
Carbon-carbon double bonds, also known as alkenes or olefins, are found in many molecules with medical and biological activity. One of the authors of the paper, researcher Richard Schrock of MIT University, shared the 2005 Nobel Prize in Chemistry for the discovery of one of the first types of olefin catalysts.
Alkanes exist either in a zigzag-like form called trans-olefin (or the E isomer), or in a U-shaped form called cis-olefin (the higher energy, Z isomer). Finding catalytic methods for the synthesis of Z alkanes, especially via olefin metathesis, has been elusive and elusive so far. Z isomers require the use of a catalyst that must be active enough to encourage the appropriate chemical reaction without damaging the cis form.
"These high-energy carbon-carbon double bonds are especially important to chemists and researchers in diverse research fields such as medicinal chemistry, chemical biology, organic synthesis and materials science," explains the researcher. "The trick here was to develop a catalyst that would be active enough to promote the Z-form alkanes, but not active enough to react with the resulting product. Olefin metathesis is a reversible reaction and there is always the risk that the reaction moves back and forth between product and starting material, a characteristic that forces the reaction to give rise to the less desirable, lower energy isomer. We have found catalysts that are active enough to initiate this challenging reaction and which are still able to not react with the resulting product and cause it to undergo isomerization (obtaining the second isomer)."
Using the common and relatively cheap metal molybdenum, the researchers showed that the catalyst is able to encourage a "cross metathesis" reaction selective towards the Z form - an olefin metathesis reaction in which two different compounds containing the alkene group are joined together to obtain a combined molecule while forming ethylene, the smallest possible compound containing the The alkane group, as the by-product. By performing their reaction in a vacuum, the team discovered that removal of the resulting olefinic byproduct can significantly increase the direction of the desired reaction while obtaining unprecedented levels of reactivity and selectivity.
The researchers demonstrated the diverse ability of the innovative catalytic method through the synthesis of a molecule that is essential for cellular function and which is related to the development of Alzheimer's disease as well as another compound used in the fight against cancer.
