Similar to the genetic "fingerprints", which are used by the police in their war against crime, deciphering the DNA profile of malignant tumors will help scientists in their war against cancer.

Similar to the genetic "fingerprints", which are used by the police in their war against crime, deciphering the DNA profile of malignant tumors will help scientists in their war against cancer. One of the biggest obstacles lies in the fact that it is not a single criminal: there are at least 200 types of cancer, and many more subtypes. The goal is to decipher the "fingerprints" of each and every one of the different types, so that eventually the cancer patients will receive personalized treatments.
Important progress towards this goal was recently achieved through a collaboration between the Weizmann Institute of Science and the Broad Institute of Harvard University and MIT. Both sides of this collaboration lead to the laboratory of Prof. Eitan Domani, in the Department of Physics of Complex Systems at the Weizmann Institute: about three years ago, his former student, Dr. Gad Getz, currently director of the Section for Computational Analysis of the Cancer Genome at the Broad Institute, began working in collaboration with Dr. Rev. Yotam Dreyer, a research student (at the time) of Prof. Domani at the institute.

The cancer genome is the DNA assembly of the malignant cell. Each type of cancer is characterized by certain defects in the genome. Dr. Getz's group at the Broad Institute, which includes about 30 biologists, biochemists, physicists and software engineers, takes part in the "Cancer Genome Atlas" project, which is managed by the US National Institutes of Health, and in other projects aimed at the same goal: deciphering the genomes of all The main types of cancer.
These ambitious projects were made possible thanks to the innovative technology developed a few years ago, high-throughput sequencing, which allows many DNA segments to be "read" at the same time. It led to a dramatic acceleration of DNA sequencing, and also to an equally dramatic decrease in its cost: from 30 thousand dollars in 1999 for a million DNA "letters", to 10 cents in 2011. As a result, scientists can now sequence quickly Hundreds of cancer tumor genomes, each containing billions of DNA base pairs. But DNA sequencing is not enough: it is no less challenging to understand what lies in this deluge of genomic information.
Dr. Getz's group at the Broad Institute is developing computational tools to analyze this information. During his visit to the Weizmann Institute in December 2008, Getz asked Dumani, his former supervisor, if any of his current students would be interested in taking part in this research. Domani recommended Yotam Dreyer. The fact that Getz and Dreyer come from similar backgrounds - both served in the IDF as part of "Talfiot", during which both received university degrees in mathematics and other exact sciences (Getz in physics, Dreyer in computer science) - ensured a fruitful collaboration.
That's what happened. Dreyer developed an algorithm called BreakPointer, which scans the entire human genome and finds the hallmarks of cancer: defects in DNA repair that lead to structural changes that differ from the normal DNA sequence. It was the first algorithm that was able to identify the exact breakpoints in DNA where the exchanges occur. "This tool is now an important and inseparable part of our effort to map all the genes and all the phenomena that contribute to cancer," says Dr. Getz.
The BreakPointer has been integrated into all analyzes of the cancer genome at the Broad Institute, and has already assisted in a number of significant scientific discoveries, including the discovery of the disturbances for which it is named: the breakpoints that lead to DNA exchanges. As reported in the journal Nature, the BreakPointer helped discover a previously unknown pattern of chromosome exchanges in prostate cancer: complex chains of exchanges within or near cancer genes. Furthermore, the scientists discovered a relationship between the location of the breakpoints and the state of the chromatin, an important component of the protective packaging of the chromosomes, indicating that genomic exchanges may be related not only to genes,
but also to epigenetic factors, that is, those that are not directly encoded in the genome.
In a study on colon cancer, published in the journal Nature Genetics, the scientists using the algorithm discovered 11 DNA exchanges that led to abnormally joined genes. Among them was a "serial" gene, which appears in a large number of different tumors - and the first gene of this type was discovered in colon cancer. Genes that are connected in an abnormal way produce abnormal proteins, and these can be a target for targeted treatments in the future, not only because they are essential for the existence of cancer, but also because they do not exist in healthy cells, so the treatment can focus on the tumor cells without harming healthy tissues.
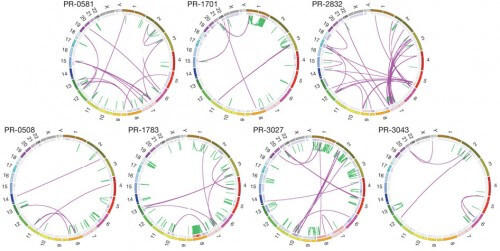
"The BreakPointer identifies the exact location of DNA breaks by locating 'suspicious' sequences in the cancerous genome, and comparing them to the corresponding regions in a normal genome," says Dr. Dreyer, currently a postdoctoral researcher at Harvard Medical School and the Broad Institute. "With the help of the algorithm, we found possible connections between DNA exchanges and other characteristics of the genome, such as mutations or the state of the chromatin." These relationships and their biological significance in different types of cancer were recently described in the scientific journal Genome Research.
The mapping of all major cancer genomes will allow scientists to better understand the molecular processes that cause cancer, thus promoting the development of drugs adapted to the unique genomic defects of each tumor. Targeted treatments of this type already exist for several types of cancer, and their number is increasing all the time. In the future, doctors will routinely map the genomes of all cancer patients, with the aim of tailoring a personal, effective and safe treatment to each one.
