I met with Inbal Sharfati-Barad to ask her what they do there at the university.
I met with Inbal Sharfati-Barad to ask her what they do there at the university.
Inbal is a PhD student in the Department of Biotechnology Engineering at Ben-Gurion University of the Negev. She works in Dr. Levi Geber's nanobiotechnology laboratory. She is married and lives in Be'er Sheva with her husband, a high-tech man. Inbal really likes to teach at the university, and in her free time she plays bridge.
Inbal, so what are you doing there?
The research in the laboratory covers a variety of fields related to the use of biological materials on a nanometer scale, and includes the use of advanced microscopy techniques. One of the main areas in the laboratory is the study of biosensors, i.e. biological sensors. My research deals with antibody arrays (immunoarray) used for substance discovery and mobile medical diagnosis.
Well, let's go in order, what are biosensors?
Biosensors are used to test the presence of a certain substance in a solution. The substance can be, for example, a poison, a bacterium or some protein, and the solution can be, for example, water, blood, etc. Hence, biosensors can be used to test the toxicity of water, to diagnose a disease, to test the blood sugar level and other things.
One of the effective tools for the realization of a biosensor are antibodies.
What are antibodies, and how are they related to medical diagnosis?
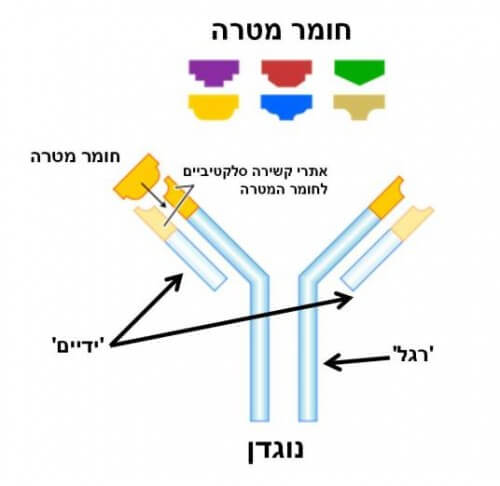
The antibody is a soldier of our immune system, and it is equipped with two hands and one leg (see Figure 1). The two hands of the antibody 'know' how to grasp only a certain substance, that is, they are adapted to bind chemically to this substance in a selective way like a unique key that opens a lock. After the antibody binds to the substance to which it is adapted, its habit signals to the rest of the immune system that a suspicious substance has been detected, and that action must be taken accordingly.
In the field of medical diagnosis, we utilize the selective binding ability of the antibody for identification and marking purposes. For example, to check the presence of a certain substance in a solution, you can use antibodies to which fluorescent markers are attached, like small light bulbs that emit light, which can be detected with the help of a suitable microscope. The antibodies will bind exclusively to the target substance, and thus we can use the microscope to check whether the same substance is present in the solution, and perhaps even estimate the amount.
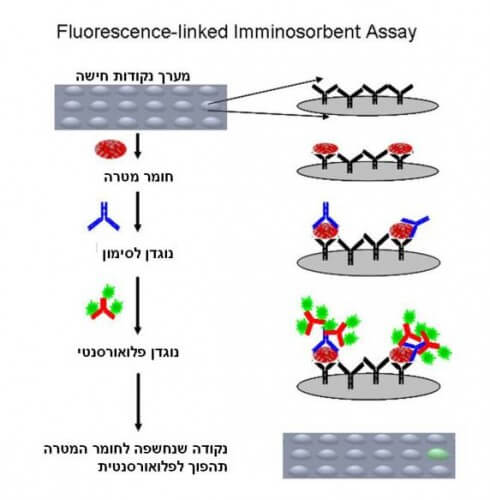
One of the most common and effective ways to use antibodies for diagnosis is with the help of special surfaces called microarrays, microarrays of binding points, and they are already in use today, for example in the blood tests we do at the doctor's. There are several ways to use arrays and antibodies for the purpose of medical diagnosis (see Figure 2), but what they all have in common is that labeled antibodies will bind exclusively to the target substance. At the end of the process, the fluorescent markers (and therefore also the target substance) can be detected with the help of a suitable microscope.
So far everything sounds great, so where is the problem?
Oh, I'm glad you ask. Anyone who has had a blood test probably remembers that the results have to wait at least a few days. One of the reasons for this is the size of a spot on the surface of the arrays. Those arrays that are in use today contain dots whose size is around 150 micrometers (micrometer = 10-6 meters, where the diameter of a hair is about several tens of micrometers). This means that only a small number of points enter the field of view of the microscope at a given moment. That is, scanning a full array takes a long time, and is performed by machines in large laboratories.
The ambition is to produce a version of the diagnostic method that is faster and cheaper and that does not need large machines and is therefore also portable, so that it can be used by operating a small device in the doctor's room.
So what is the solution?
One of the main directions being tested is reducing the size of the dots on the array to a diameter of a micrometer or less. In this way, thousands of points can be seen in the field of view of the microscope, and there is no need to scan the array. Also, this development will allow the miniaturization of the test equipment to the size of an average cell phone.
Another advantage resulting from the miniaturization is the savings in the amount of antibodies required. Since the cost of the antibodies is very high, this may also lead to a reduction in the cost of the test.
So far everything sounds great, so where is the problem?
Oh, I'm glad you ask. Minimizing the point across the diagnostic array has an unwanted side effect. The smaller the dot, the weaker the intensity of light it emits. On a small area, fewer antibodies are captured and therefore less from those fluorescent lights.
We are interested in understanding the limitations of the diagnostic system depending on the size of the dots. How much can the dots be reduced and still preserve the ability to distinguish them under the microscope, and what do the system limitations depend on?
Where do you start?
One of the known limitations stems from the microscope itself. The resolution limit of all light-based microscopes is 200-400 nanometers (nanometer=10-9 meters, the order of magnitude of a virus for example) and therefore it is not possible to measure smaller sizes. That is, even if we manage to produce a dot a few nanometers in size that shines very brightly, it will appear in the microscope to be a few hundred nanometers in size.
Another important limitation we found stems from the density of the binding sites and the intensity of the illumination.
What is the density of the binding sites?
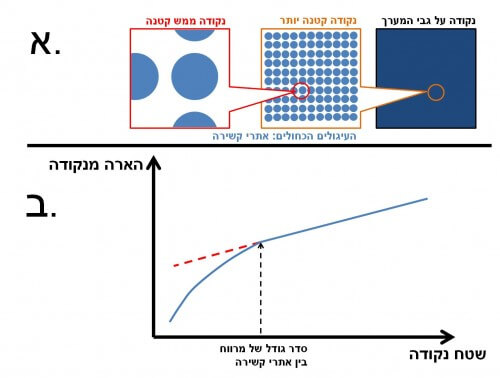
Imagine you are looking at grass from a bird's eye view. What you see is simply a green surface. But from a closer look you will notice that actually the grass leaves are scattered on the surface of the ground and are not adjacent to each other. The biological molecules bound to the surface of a point on the diagnostic array are also scattered with a certain density (see Figure 3a). As long as the spot is large, the illumination is proportional to the area. When the point is of the order of magnitude of the average distance between the binding sites, the intensity of illumination drops sharply, and is no longer proportional to the area (see Figure 3b). Therefore, using small dots requires a high density of binding sites.
To test the claim, we constructed a series of arrays with dots of different sizes, and showed the decisive effect in small dots. The conclusion is that the reduction of the dots in the array, without considering the density of the binding sites, will not lead to an improvement, but will often lead to a decrease in the quality of the detector due to a large variation in the illumination intensity of the dots.
So is it possible to reduce the dots to the size of a single micron?
During the research we showed that it is possible. For some arrays we studied we were able to detect the target material even with 300 nm dots! The problem is that we have not been able to reach a wide enough range of illumination intensity so that we can also quantify the result, that is, return an answer that is not just 'yes' or 'no', but also quantitative, that is, 'how much'.
To reach this ability we need to increase the illumination intensity of a single point, so these days we are examining several possibilities to achieve the goal. As I have already mentioned, our vision is to promote, among other things, the development of mobile medical diagnostics, as a result of which certain medical tests can be performed quickly and efficiently already in the doctor's room.
-----------------
I would be happy to meet and talk with any research student (maybe you?) who is willing to participate and tell me a little about what he is doing (and all for the price of a not too long conversation). You can contact me via Contact Us Form.
It's time to tell everyone what you're doing, maybe this time they'll understand too
The article was published on Oren Shaya's blog.to a constant extent"
