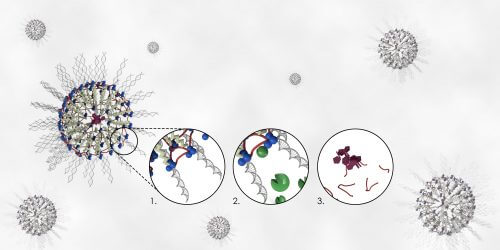
A team of researchers succeeded in developing a unique linker technology that aims to merge a synthetic drug delivery carrier (a nucleic acid nanocapsule) with a new cross-linked peptide approach. The innovative carrier makes it possible to transfer both small molecules and nucleic acids (DNA or RNA) to the various cells. This combination produces nanocapsules capable of delivering genetic or pharmaceutical molecules to a specific target within the cells or to their surface.
[Translation by Dr. Nachmani Moshe]
An innovative drug delivery system, using a combined synthetic-biological nanocapsule, could provide smart technology for targeted therapy for a variety of serious diseases. The integrated system provides a method to repair damaged cells at the genetic level - at the same time without harming the healthy cells - with the aim of increasing the effectiveness of treatments and reducing unwanted side effects.
"There is no one-size-fits-all transfer system," says Jessica Rouge, a professor of chemistry at the University of Connecticut and author of the paper detailing the findings, published in the journal Science Bioconjugate Chemistry. "The beauty of this system is that it is programmable, modular and has the ability to quickly combine a variety of peptides. It can be adjusted so that it will be able to fight new diseases in it as soon as they are discovered." The transport system combines synthetic proteins, surfactants and nucleic acids in order to create a nanocapsule that enables timed release based on the enzymes of a given drug and the sequence of nucleic acids (DNA or RNA). These findings are derived from the research of the team of scientists who tried to understand how enzymes and nucleic acids can be used in innovative ways in order to develop highly selective and targeted reactions in chemical and biological systems.
The team of researchers was able to develop a unique linking technology that aims to merge a carrier for the transfer of a synthetic drug (nucleic acid nanocapsule) with a new approach to a cross-linked peptide. The innovative carrier makes it possible to transfer both small molecules and nucleic acids, DNA or RNA, to the various cells. This combination produces nanocapsules capable of delivering genetic or pharmaceutical molecules to a specific target within the cells or to their surface. After they are routed precisely towards their destination, the substances stored inside the capsules are released in the next step, near or inside the damaged cells, depending on the biochemical environment. In the innovative system, this release does not occur unless the cross-linked peptide reacts with defined enzymes that cause the nanocapsule to cleave and eventually disintegrate within the body. While the lead researcher believes that her innovative method holds clear promise for reducing the negative side effects associated with chemotherapy, she is convinced that the method could also be applied to a number of other genetic diseases. As part of the current study, the scientists conducted tests in cell cultures using two enzymes found in high concentrations in cancer cells - cathepsin B and MMP9. When these enzymes were inserted into the innovative nanocapsules, they were able to release their cargo only in contact with the designated enzymes and under defined conditions. The researchers demonstrated that when the system comes into contact with non-designated enzymes, no disintegration of the capsule occurs, an important finding that proves that only the correct enzymatic key can release the drug stored inside the capsule. In addition, the researchers also proved that the drug is released only at a defined level of acidity, a finding that indicates that the level of acidity of the cellular environment can regulate the time and location of the release of the drug in the body.
In normal treatment methods, drug transfer is rapid and complete, and often does not depend on the levels of enzymes expressed in the cells. In addition, the existing methods increase the chance of overdose and that the drug will not reach the intended cells in its effective form. As part of such methods, negative side effects often develop that may, in some cases, be more serious than the disease itself - a phenomenon that is especially common in chemotherapy treatments, which are designed to eliminate cancer cells, but do not differentiate between them and the healthy cells next to them.
See more on the subject on the science website:
