Scientists at Stanford University have developed a battery based on the aluminum ion, which can replace most of the lithium ion and alkaline batteries in use today.
[Translation by Dr. Nachmani Moshe]
Scientists from Stanford University have developed a high-performance aluminum battery, the first ever that can also be recharged extremely quickly, both stable over time and inexpensive. The researchers claim that the innovative technology offers a safe alternative to commercial batteries that are common in the world today.
"We have succeeded in developing a rechargeable aluminum battery that may in the future replace the existing energy storage devices, such as alkaline batteries, which are harmful to the environment, and lithium ion batteries, which from time to time may catch fire. In contrast, our battery will not catch fire - even if you drill a hole through it," said Hongjie Dai, a professor of chemistry at Stanford University. The researchers describe their new invention in the prestigious scientific journal Nature.
The material aluminum (hamran in Hebrew) has been considered for many years as a tempting material as a base for batteries, this is mainly due to its low price, its low level of flammability and its high electricity storage capacity. For decades researchers have been trying, without success, to develop an aluminum ion battery that could reach the commercial market. The main challenge was to find materials that could generate enough electricity even after many cycles of charging and discharging.
Accidental discovery
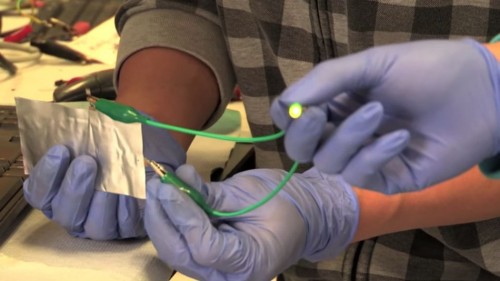
An aluminum ion battery includes two electrodes: a negatively charged anode made of aluminum and a positively charged cathode. "Scientists previously tried to use different types of materials to build the cathode," explains the lead researcher. "We discovered, completely by accident, that a simple solution to this would be to use graphite, which is essentially a collection of carbon atoms. As part of our research, we identified two types of graphite that yield good results." For the initial battery, the researchers placed an aluminum anode and a graphite cathode, along with an ionic liquid electrolyte (Wikipedia), inside a flexible cell with a polymer coating. "The electrolyte is actually salt that is in a liquid state at room temperature, so it is a very safe substance," explains the researcher. "Batteries based on the material aluminum are safer than batteries based on the lithium ion that are currently used in millions of computers and mobile phones," the researcher points out. "Lithium-ion batteries can be a fire hazard," he adds. For example, he notes, airlines in the US have banned the shipment of large quantities of lithium-ion batteries inside passenger planes. "As part of our research, we recorded videos showing that even if we drill through the aluminum battery, it continues to operate without igniting and catching fire," claims the lead researcher. "On the other hand, lithium batteries can catch fire unexpectedly - in the air, in the car or even in your pocket. Besides the high level of safety, we have also achieved breakthroughs in terms of battery performance."
One of the examples of this performance is the extremely fast charging. Smartphone users know that charging their devices, which contain lithium batteries, can take several hours. In contrast, the Stanford researchers report "unprecedented charge times" of only about one minute. Durability is another important factor. Aluminum batteries developed in other laboratories end their shelf life, usually, after only about 100 charge cycles. In comparison, the new battery was active even after 7500 charging cycles without losing its efficiency. "This is the first time that an aluminum ion battery has been produced that has an extremely fast charging time along with stability of over thousands of charging cycles," the researchers emphasize. "Another important feature of our aluminum battery is its physical flexibility - you can bend and bend it, so that it can be integrated into flexible electronic devices," notes the lead researcher. "In addition, aluminum is a much cheaper material than lithium." In addition to small electronic devices, aluminum batteries can be used to store renewable energy within the home electrical network. "The home electrical network requires a battery with a high number of charge cycles, so that it can store and release energy quickly," explains the researcher. Battery technology based on the aluminum ion also offers an environmentally friendly alternative to disposable alkaline batteries, the researcher says.

12 תגובות
Hi, I recommend a good aluminum company
Unfortunately, the commenters here are not electronics people and this platform is not so clear to them.
To our eyes, there is a fundamental difference between a super cap and a battery cap, a battery maintains its voltage almost completely until it is discharged. The capacitor breaks down according to the exponent formula, that is, from the battery voltage you can know exactly the amount of energy in it.
There is no problem adding a correlation between the capacitor and the outside world. In the circuit I developed a circuit for a supercapacitor, which has an efficiency of 96% and it corresponds exactly to the decay exponent of the capacitor.
The aforementioned development is a battery for everything and anything. And behaves like a battery. Yigal is right about the graphite electrode.. If they contact me, a flexible graphite electrode has been developed in our labs that is close to copper's hardness.
I beamed with happiness
And then I got to the comments...
I need to collect myself
Thanks Herzl, it now seems further away than I thought.
Does anyone know what the price difference is? Lithium vs aluminum
Usually if there is a significant difference then the development gets accelerated...
So I checked the original article. It really is a rechargeable battery. The main problem is that the amount of energy per unit of weight is only a quarter of a rechargeable lithium battery.
The problem is that the graphite cathode may break every Monday and Thursday when the phone is bent
I liked the article about aluminum batteries
beginning. All rechargeable batteries (perhaps non-rechargeable as well) do not have a constant output voltage during operation. Their output voltage is high when they are charged 100 percent and it drops (let's say 20 percent) when they are discharged to 0 percent.
Secondly. I don't understand microelectronics, but I don't see that there is a fundamental technological obstacle to building a battery from a large collection of tiny capacitors when each capacitor discharges separately "outside the collection" and partially discharges "into its neighboring capacitors within the collection". This allows the capacitor bank to function like a charged battery whose output voltage decreases only slightly (say a 20 percent reduction in voltage) until the bank is completely discharged. Of course, other tricks are possible to turn a capacitor into a rechargeable battery.
Of course, it is possible that the method of operation of the aluminum battery in the article is completely different from what I described. As long as there are no technical details in the publication, we will not know.
Although it is aluminum like an electrolytic capacitor that has been around for 90 years, what is described in this article is essentially a rechargeable battery and not a capacitor!
If it is indeed a capacitor then the main problem is the amount of stored energy, much less than a rechargeable battery (technical name: secondary cell).
It's not a battery, it's a capacitor and any child can build it, there are explanations on YouTube. There is nothing new here, I have a lot of them at home, I bought them on AliExpress under the name super capacitor. And it's not a simple replacement for batteries, because the receiver works differently from them. In the process of its discharge, it drops in voltage to zero, unlike a battery that maintains the voltage zone, its drop is little. Therefore capacitors are a problem, it is necessary to connect a voltage module to them.
For years, articles like this have been published as if some child invented the wheel, but this technology has been known for a long time and is also available on the market for necessities.