A new experimental approach combining chemistry and biology allows scientists to peer into the depths of the body's supreme ruler
- The mechanisms of action of the brain will be revealed to scientists only through careful investigation of individual cells combined with a large-scale review of the entire organ.
- Routine methods for optical imaging fail to penetrate deep into the brain tissue because of light scattering in contact with water and because of the fat molecules in the cell membranes.
- New methods that remove the fats and replace them with a material that holds the parts of the brain in place provide a window to peek beyond the typical barriers that prevent us from seeing the insides of the brain.
- Methods that fix the tissue in hydrogel, as these methods are called, allow researchers to examine the wiring of specific neural circuits that control different behaviors.
Our nervous system is like a tapestry, a tangled web of threads. These wires, thin fibers called axons, extend from the nerve cells and carry electrical information to other nerve cells that respond to the signals. Axons that are sent over long distances, like the hemlock threads in a weave, are woven into the "weaving threads" of the brain: axons that wind back and forth over short distances and transmit signals to perform calculations.
To understand the inner workings of the brain, scientists need to decipher how this neural tissue is organized at the level of individual components, such as individual axons. But to understand the role played by an axon, we would also like to have a general perspective on the entire brain, without ignoring the thin single axon and its contexts. But the mind is not flat like a fabric, nor is it transparent, therefore, to achieve such a point of view, a special tool is needed. However, the brain is opaque to light: fat molecules (lipids) found all over the brain, especially in the cell membranes, scatter the light sent from the imaging devices and significantly impair our ability to look beyond the outer and thinnest layer of the brain into its depths.
Now, new technology has opened up exciting new horizons for neuroscientists, creating a way to both look at the whole brain to see neural pathways and define molecular properties of individual nerve fibers that run throughout the brain. This method is based on the chemistry of water-based gels, or Hydrogels, which are polymers that form a three-dimensional network of interconnected regions and are able to contain water without dissolving. The method is used to produce XNUMXD internal scaffolds within biological tissues. In this three-step process, a clear gel is first prepared in the brains of laboratory animals or postmortem humans. The gel binds to and protects the information-rich molecular parts of the brain, including proteins and nucleic acids (DNA and RNA). After this step, the tissue components that are not of interest or that are known to scatter light, such as fats, are removed. And finally, fluorescent markers or other markers are injected into the entire brain structure. The introduction of the markers is possible because the gel is not only transparent but also designed to allow the rapid introduction of additional substances. Scientists can then shine a light on the brain through the transparent hydrogel, make the markers glow, and directly see molecules and fibers of interest in very high resolution throughout the whole brain.
This new ability to look into the depths of the supreme ruler of the body leads to many insights. Scientists are using the new approach to link the physical form and behavioral function of neural pathways involved in activity and cognition, from movement to memory. This method also helped to understand processes that contribute to the development of diseases and syndromes: Parkinson's and Alzheimer's, multiple sclerosis, autism, drug addiction and anxiety syndromes. We even helped found a company to explore applications of hydrogel tissues for cancer diagnosis. This method is now applied not only in the brain but also in various organs and tissues of the body.
See far, see transparent
Creating a transparent brain is so complicated that even evolution, over hundreds of millions of years, has not achieved this goal in large animals. Transparency can provide several important benefits. Indeed, in some biological species, a certain degree of transparency has developed during evolution that improves the adaptation of these animals to the environment (for example, as a camouflage for madmen). The blood of some fish does not even have hemoglobin, the reddish protein that gives the blood its color, and they actually exist without the blood fluid present in most vertebrates - this is how they achieve a degree of transparency. But even these animals fail to make their central nervous system transparent, despite strong evolutionary pressures. In partially transparent fish or shrimp, the nervous system is at least partially sealed. Evolution may be able to dispense with even red blood cells, but nothing seems to prevent light from moving freely through a large living brain.
The opacity to light is due to the fact that light is scattered in nerve tissue. Photons are reflected from the contact surfaces between fat and water (due to the differences in the speed at which light travels in these two substances) and in seemingly random directions (due to the structural complexity of the neural wiring). Evolution cannot easily eliminate this phenomenon. The fatty barriers serve not only as membranes for cells or internal structures in the brain, but also play a key role as an electrically insulating material, preventing leakage of the ions that transmit the electrical signals along the winding axons. Ironically, biologists need to preserve the integrity of the brain to understand it, but it is also the most difficult organ to make transparent.
In 2009, I took on the challenge of making a whole, mature brain transparent and still allowing detailed labeling of various molecules within it. At that time, hundreds of laboratories around the world had already started using the technology that my colleague and I had developed in 2004 to 2009, which allows you to activate or silence specific neural circuits in the brain using light. The method, the machine Optogenetics, combines lasers, optical fibers and genes taken from bacteria and algae that encode light-sensitive proteins called Opsins. The method makes it possible to precisely control the neural activity in specific cells in the brain of animals while they run, jump, swim, communicate or perform other complex actions. In the summer of 2009, five years after the first demonstration of the use of bacterial opsins, which took place in July 2004, most of the challenges facing optogenetics were resolved and it was possible to apply the method easily and widely. Although thousands of new insights into the neural mechanisms underlying behavior have been discovered with this method, optogenetics alone cannot provide another type of necessary information: a high-resolution image that enables an understanding of the brain-wide wiring of the single cells activated by light.
Every field of science strives to link between an overview of a system and its individual components, although this goal is often neglected (for justified reasons). Separating the components of a complex system to study them separately has always been essential to science, because removing a component from the contexts that affect it allows us to determine which of its properties are essential to it and are independent of the other components. But for a structure like the brain that has rich interconnections, decomposing the system into its elements, just like unraveling threads in a loom, is not always the best strategy for understanding the system in its entirety.
For the purposes of imaging and labeling components, the opaque nature of mature mammalian brains has always required dissection of the brain, usually through sections, so that in fact the three-dimensional structure became hundreds or thousands of two-dimensional sections. This process consumes enormous amounts of time and money, especially when many brains are required to produce a statistically significant result (as is often the case when studying mammalian behavior). Moreover, vital information is lost forever. Since with the help of optogenetics a new use of the brain in its entirety was already possible, in 2009 I began to wonder what else could be built in the brain to help us with the problem of light scattering.
The core of the idea was buried 15 years before. In the mid-90s, I was intrigued by the idea of trying to build brain-like circuits in the lab, starting with single cells. One way to do this may be through the seeding of neural stem cells on polymeric scaffolds, and there on these scaffolds it will be possible by biochemical means to induce the cells to become neurons. During these attempts, I delved into the scientific and engineering literature of hydrogels that seemed to be particularly successful as scaffolds, being suitable for biological tissues and also transparent.
In the years that followed, I ended up only doing simple preliminary experiments where I seeded cells on polymer scaffolds and turned them into neurons, but I never got to the stage where I created a complete brain-like structure from single cells—a very challenging task. Even so, I made sure to carry the dusty binder, which was labeled "Hydrogels" and contained tightly stapled pages, with me as I moved from lab to lab over the next 15 years, and from one stage to another in my career (receiving a doctorate in neurobiology in 1998, completing my residency in psychiatry and postdoc, and launching my engineering lab at Stanford University in 2004). But the basics of the plan were already in my mind, and the idea took root and eventually developed, with the help of some incredibly talented people in the lab, into a working strategy for building a transparent and accessible brain.
A sketch I scribbled in February 2010, while sitting at my desk after a long period of thinking about imaging of the whole brain, describes the basic idea [see photo]. The idea was indeed based on the old principle of building a brain from single cells, but the innovation was to apply it in the opposite direction: instead of starting from a hydrogel and building a brain inside it, it occurred to me to start from a whole brain and build a hydrogel inside it. The hydrogel will serve as a supporting structure that will preserve the spatial location of the brain components that are important to us, such as proteins and nucleic acids, but will allow the removal of anything else that prevents us from viewing the depths of the brain. At the same time, the hydrogel will prevent the brain from collapsing into a shapeless mass while dissolving or digesting less interesting components.
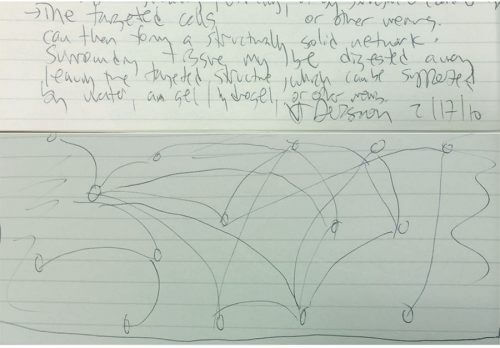
The first experiments, which bridged separate fields of science and gave tentative shape to what was previously only an idea, can only be optimally evaluated over the years when we have enough perspective to examine them. Two brave and creative researchers who were in the laboratory at the time - Viviana Gardinero and the director of the laboratory Charo Ramakrishnan - were the first to agree to take on this difficult project. The risk was so high that I decided not to involve the whole group. I thought that these two experienced researchers (who had already been very successful in other projects) would be able to handle the risk and the disappointment in case of failure.
At the beginning of 2010, Gardinaro and Ramakrishnan began to examine how to protect nerve cells from substances that can destroy the structural details of the tissue and the cell membranes. Theoretically, filling the nerve cells with some resistant polymeric material could provide the protection: the nerve cells should remain intact in the support of the hydrogel. The two tried several strategies, including inserting genes that code for certain enzymes that would allow the nerve cells to produce resistant polymers such as chitin or cellulose. It turned out that the best approach, which arose from Gardinaro's creative idea, was to produce inside the cells another biological polymer, Keratin. She showed that in the nerve cells in culture, keratin can protect the structure of the cell and the cortex in the whole brain (when the neurons are stabilized with keratin and hydrogel that adds external support), it will be possible to wash out the lipids with detergent, and reveal the desired brain structures, which are hidden inside the transparent hydrogel .
At the time, building a hydrogel in the whole brain was just an idea. I decided to speed up the project with the help of a chemical engineer. Although no one outside the lab knew about the project, I scoured my inbox for inquiries from postdoctoral candidates who might have a suitable background in hydrogels. In the search, the name of Kwangon Cheng, a highly accomplished chemical engineer then working at the Georgia Institute of Technology. Cheng heard about our work in the field of optogenetics and stem cells, and asked to join the lab.
In early March 2010, a few weeks after I sketched the original illustration of the hydrogel idea, I had my first brief phone call with Cheng while I was in a meeting in Utah. And then I did something I hadn't done before (or since) because I was so convinced of the new research direction. I invited Cheng to join the lab without a lab visit or even a face-to-face interview. Strange times for the neurobiology lab: a chemical engineer pops out of nowhere.
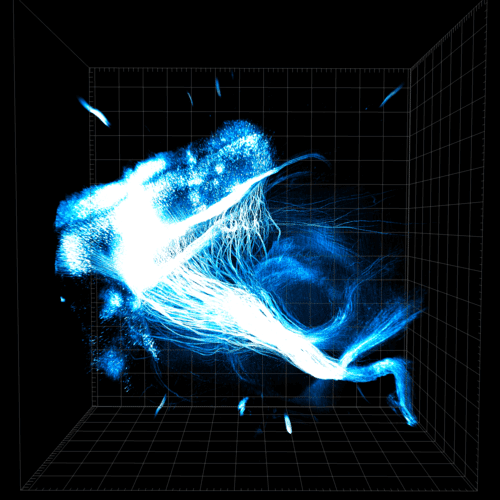
Upon arrival, Cheng immediately dived into the secret project. By the end of 2010, the three members of the group had created transparent cubes of mouse brain in my laboratory in which it was possible to clearly see, inside the hydrogel, brain cells preserved with the help of keratin, even at a depth of hundreds of microns inside the tissue, much deeper than it was possible to reach with existing methods [See illustrations]. The first functional hydrogel produced by Cheng was based on Acrylamide, often used in laboratories to separate nucleic acids or proteins. These tissue-gel hybrids were designed so that we could inject fluorescent and other markers directly to see conserved proteins and structures like axons. After a large number of labeling cycles we discovered that we do not need keratin at all to keep the cell components in place: the hydrogel alone was enough. Despite the pioneering work in other approaches that they didHans-Ulrich Dot וAtsushi Miyawaki (Methods 3DISCO and- Scale respectively), such transparency and accessibility in the mature brain of a mammal has not been achieved so far.
The hydrogel-tissue methods allow access to the depths of the brain, and provide insights into the biology and diseases of the brain.
This version of the hydrogel-tissue based on acrylamide (there are currently many other versions published) was called CLARITY, sort of an acronym for Full name. Since we published the method in 2013, even this single version of the method has been adopted for a variety of basic science as well as clinical applications (e.g., in postmortem examination of the brains of people with autism or Alzheimer's), as well as in the spinal cord and brain of mice (e.g., to discover pathways neurons that control the behavior of fear and anxiety that were not known before). Laboratories around the world have already published many articles on studies in which they used this method to understand the basic structure of the nervous system, often in combination with optogenetics, and to come up with new ideas for understanding normal and disturbed neural pathways.
Just as during the first five years of the optogenetics method using bacterial opsins, scientists developed a variety of innovations that enable wide-scale applications of the method, so the method for building a hydrogel inside the brain advanced dramatically in the first years after its development. For example, the earliest version of the hydrogel method involved a step in which an electric field accelerates the removal of charged detergent particles bound to lipids. This step required skill and sometimes, if the tension was too high, the tissue was damaged. The problem was solved Raju Tomer, Brian Sve וLi Ye, who were all in my lab at the time, who published in early 2014 a simpler version of this step in two papers (one in collaboration with colleagues from Sweden). The method has since become known as passive CLARITY, because it does not include electric fields. Tomer and the group also described a special hydrogel-brain imaging method that uses a fast, high-resolution form of microscopy called Light surface microscopy, which was adapted to meet the challenge of rapidly imaging large gel volumes by scanning surfaces instead of light spots.
At the time, both Gardinero and Cheng already headed their own successful laboratories (at the California Institute of Technology and the Massachusetts Institute of Technology, respectively), and each produced important innovations. Soon further developments came from many other researchers as well. Gardinero has independently developed and published a whole-organism CLARITY strategy named PARS. Gardinero and Cheng separately published new hydrogel formulations called PACT and SWITCH respectively, and now laboratories around the world have already described a wide variety of hydrogel-tissue compositions. But so far we have only begun to scratch the surface of the experimental field of exploring possible hydrogels. In 2013 Cheng and I published a very long list of possible hydrogel compositions, mAcrylates Committee elgitnates and beyond that. In my labs and those of my colleagues, we are currently looking at ways to make the polymers even more active, for example introducing chemical elements that will cause adjustable electrical conductivity or chemical reactivity, properties that will open up new possibilities for us.
Another challenge focuses on the properties of hydrogel-tissue conjugates, which, as we described in our 2013 and 2014 articles, cause the tissues within the hydrogel to expand considerably. The phenomenon does not pose a difficulty in all cases and does not interfere with high-resolution imaging, neither with the original CLARITY method nor with later methods (PACT/ePACT in 2014, then ExM/proExM and MAP in 2015 and 2016) developed by other groups as well cause swelling of the tissue. But in order for us to be able to compare the transparent brains we created with academic atlases of the brain, it is necessary to accurately preserve the original tissue. Therefore, we developed a final, possible step to shrink the tissue back to its original size.
With the help of her and another team member, Will Allen, developed a laboratory and published fast and automatic data visualization and analysis software that can be freely downloaded and used. my colleague's group, Mark Tasya-Lavain, then at Rockefeller University and now president of Stanford University, did so for the method ofiDISCO which she developed. These two supplementary articles have been published In the same issue of the journal Cell In June 2016. My group, and in it Emily Silvestrek, Priya Rajasthopathy וMatthew Wright, also managed to enable the reliable use of a particularly important type of simultaneous fluorescence labeling of a large number of RNA molecules in the whole brain by using a different type of hydrogel, as we reported in an earlier article Which was published in Cell in March 20166.
"After creating a transparent brain, our group could look at a region called the prefrontal cortex and see how populations of cells that mediate positive or negative experiences are wired differently."
The ability to label several types of molecules, including nucleic acids such as RNA, has become a unique advantage of the hydrogel approach and opens up extensive possibilities for studying gene expression. And after solving all these challenges, many of them only this year, the method matured and reached the stage where it is applied in many laboratories around the world.
tying the ends
It's amazing to look back and compare the modest video from 2010 and the fully functional implementation, just six years later. An important goal in the progress of the hydrogel vision was to connect the two methods: optogenetics of the whole brain and obtaining structural information of the whole brain. This goal was achieved and published in several articles, including In a June 2016 article in Cell. The research described in this article focused on the prefrontal cortex, an area responsible for regulating complex cognitive processes and emotions. Scientists hope that understanding how this structure controls such a wide range of behaviors could provide insights into psychiatric conditions such as autism and schizophrenia.
My group members, Yeh, Alan, and Kim Thompson, in collaboration with colleagues from other laboratories, including the laboratories of Lycon Low וJennifer McNab, both at Stanford, used optogenetics for the first time to define a group of cells in the prefrontal cortex that is active during rewarding experiences such as particularly tasty food or even cocaine (and also controls the appropriate behavioral response). We then discovered a complementary population of cells in the prefrontal cortex that are active during negative (unpleasant) experiences. And finally, using our state-of-the-art hydrogel methods, we were able to show that these two cell populations are wired differently throughout the brain. The cells associated with positive things send extensions mainly to an area deep in the brain called nucleus accumbens [see figure], while the cells associated with negative things are connected to another deep area called The venola is lateral. In this way, the hydrogel and optogenetics approaches allow scientists to study biological tissues in their natural state in sequential ways not previously possible, advancing the understanding of basic biology and health and disease.
The full appreciation of complex systems emerged at the same time as the ability to exchange information both on a local scale and on a broad scale, whether it is the whole brain or other complex tissue. In neuroscience, it is now possible to collect vast amounts of information, including rich and diverse details that illuminate the structure of the entire brain, its molecular components and cell activity. As a result, we begin to form a broad yet detailed view of the brain.
It is difficult and rare to achieve such a broad point of view that also includes a local resolution, but it is important to respond to the challenge. The properties of complex systems often originate in local interactions, like the interwoven threads of a tapestry and like the scientific process itself. Only with the help of an overall perspective can we understand the role played by each thread in the fabric.
