A new study examines how inflammatory cells cause precancerous processes in the cells next to them. Its application may help prevent tumors
Merit Sloin
Direct link to this page: https://www.hayadan.org.il/memet270804.html
Cancer researchers are returning to a theory that has been neglected for many years. About 150 years ago, the pathologist Rudolf Wirkov, who relied on pathological findings he diagnosed, claimed that chronic inflammation causes cancer. Today, most researchers working in the field believe that such a connection does exist. For example, most cases of liver cancer develop as a result of chronic inflammation of the liver, caused by infection with the hepatitis virus (infectious jaundice of the liver). Chronic inflammations of the stomach and colon also sometimes lead to the development of malignant tumors. It is estimated that about 20% of all cancer cases develop as a result of chronic inflammation.
Researchers have tried to understand how inflammatory processes cause cancer. A team of researchers from the Hebrew University and the Hadassah Ein-Karem Medical Center discovered the factor responsible for this connection. The findings of their research were published yesterday in the online edition of the weekly "Nature", in preparation for their future publication in the regular edition.
Inflammation is the body's normal response to dangerous situations - invasion by a foreign agent, contamination with chemical substances, exposure to toxins, and more. In all these situations, the inflammatory cells are mobilized to eliminate the offending factor. In most cases the inflammatory process is limited and ends with healing, but sometimes it gets out of control and becomes chronic. In these cases, the danger of developing cancerous tumors is great.
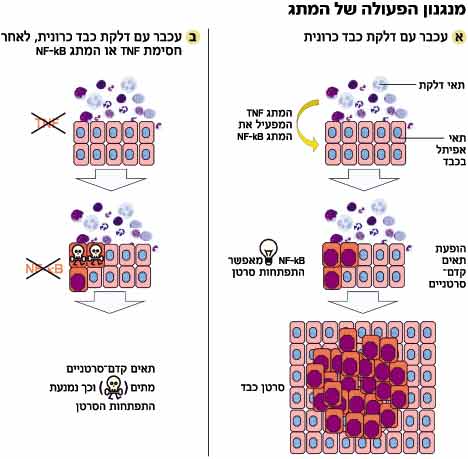
In areas affected by chronic inflammation, the inflammatory cells are in close proximity to epithelial cells (covering cells). This proximity causes the formation of processes in the epithelial cells that do not occur in a normal state. In a normal state, the epithelial cells (like most body cells) go through life cycles of division and proliferation on the one hand and death on the other, thus maintaining their constant number in the body.
But in the cells next to the inflammatory cells, the balance is disturbed - the number of dividing cells exceeds the number of dead cells. The excess cells continue to accumulate, a condition known as "premalignant". The process is accompanied by mutations, which damage the natural control mechanisms present in each cell, whose role is to protect against the formation of malignant tumors. The appearance of more and more mutations creates a developed cancer.
Due to the proximity and contact between the inflammatory cells and the epithelial cells, the team members hypothesized that the inflammatory cells transmit a special signal to the epithelial cells - which causes changes within them and makes them prone to developing cancer. "In order to identify the change, we looked for a suitable candidate," says Prof. Yanon Ben-Naria from the Lautenberg Center at the Hebrew University School of Medicine, who conducted the study together with Dr. Eli Pikarski from the Department of Pathology at the Hadassah Ein-Karem Medical Center and a team of researchers.
The choice fell on the protein NF-kB (kappa NF-B), which was discovered about 20 years ago by Nobel laureate David Baltimore and turned out to be a central control factor in the living cell. The protein is actually a switch in the cell. It switches to an active state (on) in the cell nucleus, when it receives signals from the cell. When a violent bacterium attacks the cell, a signal chain is immediately activated that transmits the information about the attack to the cell nucleus, where a command is given to activate genes that produce proteins that fight the bacterium - for example, antibodies. NF-kB is found in the nucleus at a central junction, which channels many signals coming in and out of the cell.
"We know that NF-kB is the main factor that activates reactions of the immune system and is sometimes activated, for an unknown reason, in certain types of cancer. However, it was not clear whether its activation is the cause of the cancer process or a result of it," says Pikarski. "The knowledge we gathered led to the assumption that with a relatively high probability, NF-kB may be the candidate that switches to the on state in the epithelial cells, following the contact with the inflammatory cells, and may be responsible for the mechanism that changes the balance between the proliferation and the death of the epithelial cells."
To test their hypothesis, the researchers created a strain of genetically engineered mice in which the NF-kB switch is turned off. In this situation, the inflammation continues, the friction between the cells is constant, but the switch does not activate genes, Pikarski explains. "The results showed that we were able to reach a renewed equilibrium between the proliferation of the epithelial cells and their mortality. In the mice in which they turned off the switch, the death of the epithelial cells increased and most of them did not develop tumors."
The next question the researchers faced was who operated the switch. "The data led us to the TNF alpha protein, which is released following inflammation and transmits messages between cells. The substance is known and antibodies that neutralize it are used in the ongoing treatment of various inflammatory diseases," says Ben-Naria. "To check if it indeed activates NF-kB, we injected mice suffering from chronic hepatitis with an antibody that neutralizes TNF alpha. In this way, we created a situation where the ignition remains intact, and all that remains is to check whether the switch was turned on or not." It turned out that the switch was not turned on and as a result the precancerous cells that started to form died one by one. "It was a major blow," says Ben-Naria, "now we know who is responsible for the development of cancer in hepatitis and who activates it."
The discovery may have applied medical implications. "Today, in many patients, there is no way to prevent chronic inflammation and there is no known way to prevent the cancerous process," says Pikarski. "Following our findings, we think it is possible to reverse the process at an early stage when the epithelial cells are still in a pre-cancerous state. At this stage, it is possible to intervene using drugs based on antibodies against the TNF alpha protein, which will block its activity and thus return -kB NF to the off state. Treatment with substances that block TNF alpha will mean that every time the pre-cancerous cell raises its head, we will cut it off and create a situation where the tumor will remain in its initial state, which is not harmful."
The researchers emphasize that the results obtained in mice are indeed encouraging, but additional experiments must be performed in mice in order to confirm them. To implement the treatment in humans, controlled clinical trials will be required. The research, which lasted four years and cost 400 thousand dollars, was supported by the National Science Foundation and by a private foundation designed to promote research in the treatment of prostate cancer.
Yadan engaged in cancer research
https://www.hayadan.org.il/BuildaGate4/general2/data_card.php?Cat=~~~935021620~~~178&SiteName=hayadan
