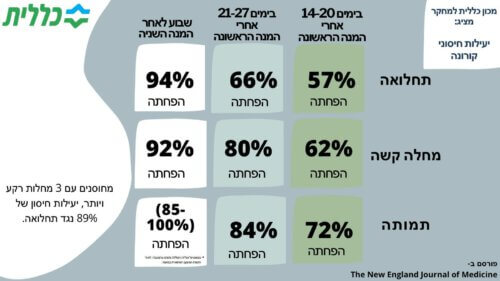
Final results of the Klalit Research Institute regarding the effectiveness of corona vaccines are published in the leading medical journal in the world - The New England Journal of Medicine: After the first vaccine dose: 57% effectiveness in preventing morbidity and a 62% reduction in severe disease After a second vaccine dose: 94% reduction in morbidity and 92% in severe disease
The largest study of its kind about the effectiveness of the vaccine against Corona conducted by the Klalit Research Institute is published in the leading scientific journal The New England Journal of Medicine. The extensive study that included over a million people, the first of its kind to be published in global scientific literature, was conducted at the Klalit Research Institute in methodological collaboration with researchers from Harvard University.
The research was conducted by Dr. Naa Dagan, Dr. Noam Barda, Dr. Eldad Kaptan, Oren Miron, Shay Partchik, Prof. Mark Katz and Prof. Ran Blitzer from the Klalit Research Institute, as well as Prof. Miguel Hernan and Prof. Mark Lifshitz from Harvard University School of Public Health, and Prof. Ben Rice from Boston Children's Hospital and Harvard Medical School.
The study looked at data on about 600,000 people who were vaccinated in Israel, against about 600,000 unvaccinated people who formed a control group. The study provides for the first time a wide-ranging picture, using an advanced research method that has been peer-reviewed, about the effectiveness of the vaccine against the corona virus in the context of mass vaccination - after the first dose and after the second dose.
The results show that two weeks after the first dose, a 57% reduction in morbidity, a 62% reduction in severe disease and a 72% reduction in mortality was observed. 21-27 days after the first vaccination (a period during which in the vast majority of cases the second vaccine dose was also given) a reduction of morbidity by 66%, a reduction of serious illness by 80% and mortality by 84% was observed. After full vaccination, that is, one week after the administration of the second dose of the vaccine, the morbidity rates from corona decreased by 94% and the rate of severe disease by 92%, as already published in Israel.
The size of the sample made it possible to go down to a high level of detail of subpopulations
The size of the sample made it possible to evaluate the effectiveness of the vaccine for specific subpopulations as well. In terms of age groups, the effectiveness of the vaccine was found to be similar in the different age groups, including at the age of 70+. Among the subgroup of vaccinated people with 3 or more underlying diseases, it was found that the vaccine reduces morbidity from corona by 89% compared to 94% in the general population.
During the entire study period (until the beginning of February 2021), 10,561 cases of corona were recorded in the study population, of which 5,996 were symptomatic, 369 were hospitalized, 229 were severe, and 41 were fatal.
According to Prof. Ran Blitzer, Director of the Klalit Institute for Research and the Klalit Innovation System, "The research shows unequivocally that the vaccine is highly effective in preventing illness and severe illness from the corona virus one week after the second dose, and provides only partial protection in the earlier weeks after the vaccination. These results are similar to the results reported in the original Pfizer clinical study. The practical significance of the study is clear, and I urge everyone who has not yet been vaccinated to do so. The results correspond well with the latest trends in morbidity in Israel - a continuous decrease over the past month in the rate of hospitalization and the rate of severe morbidity in the older age groups that were vaccinated first, and a similar picture three weeks later among the younger age groups, where vaccination only began a few weeks later.
The study was conducted from the beginning of the vaccination campaign in Israel on December 20, 2020 until February 1, 2021. In this period of time, the third wave of the corona also occurred, during which the British variant gradually became the dominant strain in the country.
Prof. Ran Blitzer
Publication in the strictest medical journal
Prof. Blitzer adds, "Due to the methodological complexity of evaluating vaccine effectiveness from retrospective data, we carefully analyze the data under constant peer control by the world's leading scientists in the field, and while performing complex sensitivity analyzes to faithfully assess the effect of the vaccine in different subgroups of the population. Now the results and methodology have received the approval of the leading and strictest scientific body in the world - the world's leading scientific journal in medicine - the New England Journal of Medicine. Now the findings are clear - the vaccine is highly effective and significantly reduces the morbidity and mortality from the disease, but the protection is not absolute and not close to 100%. Therefore, in this period of widespread spread of the disease in the community, the vaccinated must show extra caution - even after a week has passed since the second dose and certainly before."
The extensive size of the current study allows for a detailed evaluation of the effectiveness of the vaccine in preventing a wider range of outcomes (documented infection, morbidity, hospitalization, severe morbidity and mortality), over different periods of time and in subgroups of the population. The study was conducted from the beginning of the vaccination campaign in Israel on December 20, 2020 until February 1, 2021. In this period of time, the third wave of the corona also occurred, during which the British variant gradually became the dominant strain in the country.
The partnership with Harvard
Prof. Mark Lifshitz, director of the Center for Infectious Disease Dynamics and a professor at the School of Public Health at Harvard University, who participated in the research, said that "in all studies on vaccine effectiveness, a key challenge is to ensure that the groups we compare to detect the impact of the vaccine are similar in characteristics that may predict whether they will become infected or will get sick This is an extremely complex study to conduct while a vaccine operation is progressing rapidly. Klalit's extraordinary database made it possible to design a study that addressed these challenges in a way that provides great confidence in the study's conclusions."
The matching between the study groups was performed based on an extensive array of demographic, geographic and health characteristics related to the risk of serious illness, health status and healthy behaviors. Allocation to study groups was performed dynamically based on daily vaccination status, and approximately 85,000 individuals moved from the unvaccinated group to the vaccinated group during the study period. Sensitivity analyzes were conducted to ensure that the estimated vaccine efficacy is not affected by possible biases.
The virus knows no borders
Prof. Ben Reiss, Director of the Predictive Medicine Group at Boston Children's Hospital and Harvard Medical School, stated that "Israel's impressive vaccination campaign was a rare opportunity to measure the effects of vaccination in the real world. The global scientific effort that enabled the development of vaccines in record time continues now with international collaborations focused on evaluating vaccine efficacy. The virus knows no borders, and neither do the scientists fighting it. This is the way to do science.”
Prof. Miguel Hernan from the School of Public Health at Harvard University, who was a partner in the study, said that "This study is a perfect example of how clinical trials and data-based research in the field of health complement each other. The original trial of the Pfizer-Biontech vaccine provided convincing evidence of its effectiveness in preventing symptomatic infection, but estimates of its effectiveness against severe disease and in specific age groups were not precise enough. This analysis of Klalit's qualitative database is similar to the original trial design and confirms the effectiveness of the vaccine in serious diseases and in different age groups. This combination of evidence from randomized and observational studies is a model for effective medical research, something that is especially important during the Corona period."
More of the topic in Hayadan:
