The research published in PNAS - the journal of the National Academy of Sciences in the United States - was led by Prof. Reut Shelgi, research colleagues Niv Sabat and Falonia Levy-Adam and doctoral student Amal Younis from the Rapaport Faculty of Medicine

Technion researchers present new findings concerning the gradual damage to the protein control system during the aging period. the study published inPNAS - the journal of the National Academy of Sciences in the United States - led by Prof. Reut Shelgi, research colleagues Niv Sabat and Falonia Levy-Adam and doctoral student Amal Younis from the Rapaport Faculty of Medicine.
The research focused on an essential control system that protects the cell from the accumulation of disordered proteins; When this system is damaged, a deposit of such proteins accumulates in the cell, and this accumulation has a toxic effect, especially when it occurs in the brain.
Proteins are active partners in all biological events inside and outside the cell, and their function depends on their success in folding into a specific three-dimensional structure that allows them to have proper interactions with other components in the cell.
In many cases, the proteins are misfolded, which not only prevents them from fulfilling their role, but also causes them to become "sticky" and accumulate in the cell into a toxic deposit. This deposit may damage cells in general and brain cells in particular in the process involved in the development of Neurodegenerative diseases including Alzheimer's, Parkinson's, ALS and Huntington's.
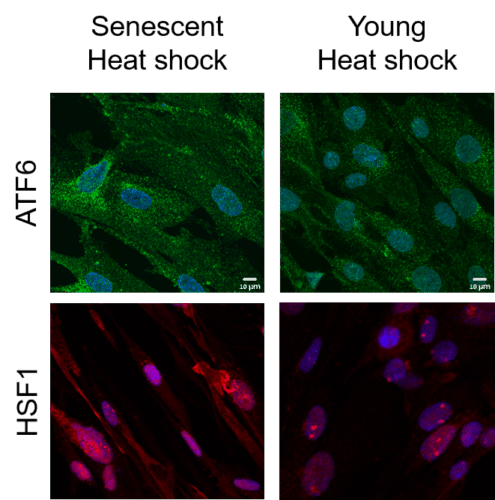
Since the formation of disordered proteins is a common event, a cellular control mechanism was developed during evolution that examines the quality of the protein from the moment of its formation in the ribosome until its death. This mechanism, called proteostasis, locates disordered proteins and treats them in one of three ways: refolding them in the desired way; their isolation and neutralization in a way that will not affect cell activity; Or that it causes the proteasome, the cellular garbage can. This mechanism makes sure that the disordered proteins, which are also formed in the healthy person, are treated and do not accumulate into the same toxic deposit that leads to the formation of neurodegenerative diseases of the brain. However, the aging process involves the weakening of the proteostasis mechanism. This weakening, previously demonstrated in worms (nematodes), is now demonstrated for the first time in aging human cells. This is through the characterization of transcription, RNA processing and protein synthesis with genome-wide tools.
In the article in question, the Technion researchers present the aforementioned phenomenon in the context of heat stress. They showed that unlike the young and healthy cells, in the old cells the response to heat shock is insufficient, and this is due to the deterioration of the proteostasis mechanism. In contrast to the young cells, the old cells do feel the stress but fail to activate the adaptive transcriptional response necessary to fully overcome the stress. The researchers also found that the activity of the proteasome, the "cellular garbage can", deteriorated in the old cells and did not return to itself even after they were given time to recover from the shock.
Prof. Reot Shelgi completed a bachelor's degree in biology and computer science at Tel Aviv University and a master's and third degree in genetic expression research at the Weizmann Institute. In October 2014, after a post-doctorate at MIT, she joined the faculty of the Rapaport Faculty of Medicine. Today she heads a laboratory that combines computational biology and molecular biology and is a member of the Rapaport Institute and the Prince Center for the Study of Neurodegenerative Diseases.
for the article inPNAS click here

One response
The explanation under the pictures confused you.