New type of fuel cells, able to provide high performance electricity, while operating at high temperatures

Fuel cells are more efficient than normal battery cells, because they can be charged quickly and release only clean by-products - water. Thus, fuel cell technology is a promising green technology with applications in automotive, home appliances, as well as space industry and marine applications.
An important step in the development of this environmentally friendly technology was recently made by the research group of Prof. Dario Dekel from the Technion, with the assistance of a research grant from the National Science Foundation. Prof. Dekel and his team developed a fuel cell based on an "anion" exchange membrane, which is ions charged with a negative electrical charge, (AEMFC) operating at high temperatures. As in an electrochemical cell, a fuel cell includes electrodes (cathode and anode, where redox reactions occur); and a polymer electrolyte membrane, which allow the flow of ions between the electrodes. Unlike conventional electrochemical battery cells that use metallic ions that are stored for operation, a fuel cell operates on a continuous supply of fuel (such as hydrogen, methanol, etc.), and oxygen (from the ambient air). If the supply is maintained, the fuel cell continues to operate. Typical electrochemical reactions that occur in fuel cells are the oxidation of hydrogen at the anode and the reduction of oxygen at the cathode.
The rate of these electrochemical reactions is very low, so metallic catalysts made of precious metals (such as platinum and palladium) must be used. The common type of fuel cells is based on proton exchange membrane (PEMFC), phosphoric acid fuel cells, alkaline fuel cells and more. Recently, a subcategory of alkaline fuel cells, based on anion exchange membrane (AEMFCs), has been developed, in which a polymeric membrane is used to transport hydroxide ions (OH-) (a diatomic anion of oxygen and hydrogen) between the electrodes. These fuel cells are superior to conventional fuel cells because they use inexpensive metal catalysts, such as iron and nickel, instead of expensive platinum, thus significantly reducing the cost of the product. In addition, an AEMFC can operate with a wide variety of fuels, such as nitrogen-based fuels, which cannot be used in a PEMFC. Anion exchange membrane fuel cells are able to perform long-term operations because they contain very stable materials in the working cell environment. In recent years there has been a significant development in the AEMFC technology in terms of OH conductivity- which led to an increase in the performance of these fuel cells. However, the operating temperature of these cells was quite low (40-80 degrees Celsius), despite the need for an efficient fuel cell operating at higher temperatures.
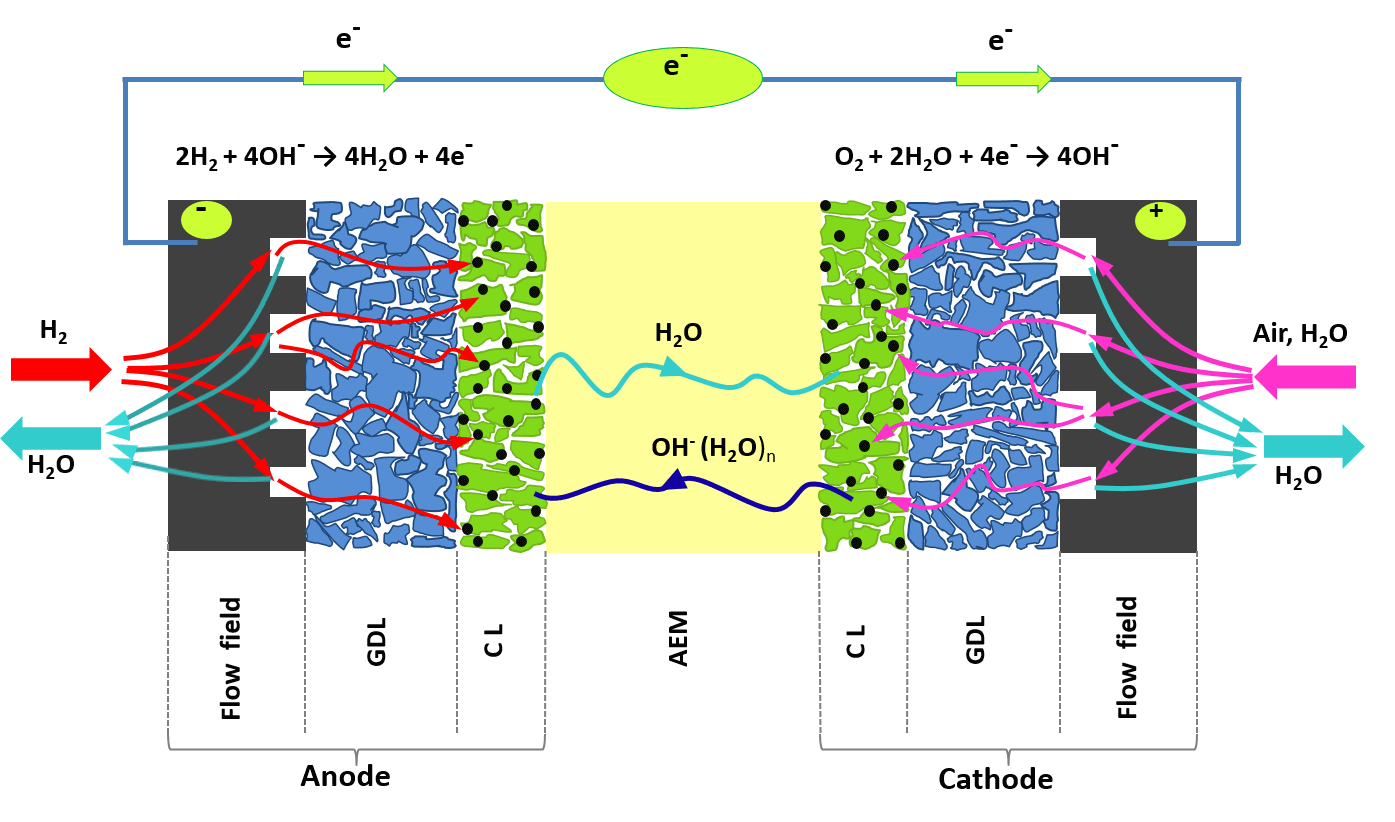
Prof. Dekel and his team recently succeeded in developing fuel cells based on a high temperature anion exchange membrane, capable of operating at temperatures of more than 100 degrees Celsius. The anion exchange membrane (AEM) tested at 110 °C showed a high conductivity of the hydroxide (OH-) which was close to 300 millisiemens per cm (Siemens is the unit of electrical conductivity), much higher than previously reported. A method previously developed by Prof. Dekel's group ("off-site"), was designed to measure the true hydroxide conductivity at high temperatures. This method involves applying an external electric current through the membrane, to force the release of the large anions in the form of carbon dioxide from one electrode with the help of the in-situ formation of (OH-) at the opposite electrode. The current is turned on continuously until all the anions in the membrane are exchanged for OH-.
This method is easy to implement and provides environmental conditions similar to a fuel cell in working condition. The anion exchange membrane of fuel cells at high temperatures developed by the research group at the Technion showed high performance: the fuel cells maintained a performance of 0.8 volts after 50 hours of operation at a constant current density of 200 milliamperes per square meter. This discovery of the possibility of operating the anion exchange membrane of fuel cells at high temperatures opened a new field of research and set a significant milestone in the technology of fuel cells based on anion exchange membrane.
The research group led by Prof. Dekel, in collaboration with Prof. Brandon from the Technion, also designed a computational model that allows predicting the performance stability of fuel cells based on anion exchange membranes. The model takes into account various cell parameters, such as chemical decay of the membrane, its dependence on the level of hydration, cell material properties, as well as design factors and operating conditions, to predict the performance stability of the cells. This model allowed the researchers to discover the importance of the operating current density and membrane properties on the performance of the fuel cell and its lifespan. The model also predicted a significant improvement in the stability of these fuel cells, with theoretical lifetimes in excess of 5,000 hours being achieved, based on the use of thinner membranes and materials with reduced decay kinetics. Applying these conditions during the development of this type of fuel cells may satisfy the fuel cell's longevity requirements, so that they can be used, among other things, to power cars.
Life itself:

Prof. Dekel likes to play chess. travel, and meet people from different cultures.
More of the topic in Hayadan:
