Researchers predict the existence of a "super-solid" with heavy hydrogen
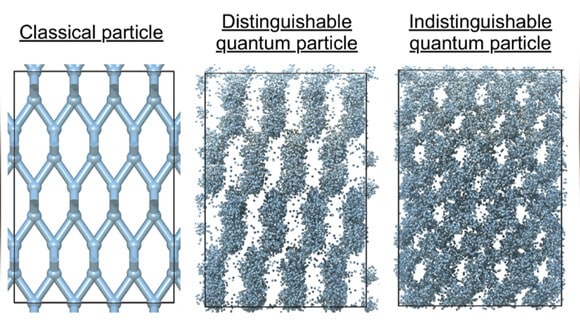
How can matter be both solid and flow without friction? With the exception of the states of aggregation that are familiar to most of us, solid, liquid and gas, there are also exotic and particularly fascinating states of aggregation that only exist due to the modified properties of quantum particles. Supersolids are such materials, and they have two properties that sound completely contradictory: a structure with a structured and fixed order, and the flow of the material without friction. A new study with the participation of a researcher from Tel Aviv University presents a theoretical prediction that deuterium, an isotope of hydrogen that is a component of heavy water, can acquire this property at extremely high pressures and low temperatures.
The property that makes a material 'superfluid'
From the findings of the research it appears that the deuterium, which appears as a gas at room conditions, is able to change the aggregation state to a "super-solid" when it is at a pressure of 8 million atmospheres and close to absolute zero temperature. The research was conducted in collaboration between Dr. Chang-Wu Myung from the University of Cambridge, Prof. Michela Prinlello from the Italian Institute of Technology in Genoa and Dr. Barak Hirschberg from the school of chemistry at the Faculty of Exact Sciences by Raymond and Burley Sackler. The study was published in the prestigious journal Physical Review Letters.
The existence of supersolids was predicted more than50 years at the theoretical level and ignited fierce controversy among physicists. However, about five years ago its existence was unequivocally proven in experiments on cold trapped atoms. The researchers say that understanding the mechanisms underlying the process and discovering new supersolids is a research topic that has gained a lot of momentum in recent years, and even more so since the experimental observation.
"In order for a material to flow without friction, what is known as 'superflowing', the wave functions of the atoms that make it up must overlap so that it is no longer possible to distinguish between them. And if so, the question arises - how is it possible for them to maintain the spatial order that characterizes solids?" explains Dr. Hirschberg.
"The reason is this: in the research we discovered that the high pressure forces the deuterium atoms to come closer and become a solid with the properties of a metal. In a metal, which is synonymous with a conductive material, the electrons of the deuterium atoms repel each other a little less than in an insulating material, allowing the atoms to get even closer. These two properties together, encourage the formation of the superflow. In addition to that, we showed in computer simulations that deuterium becomes superfluid, but also maintains its crystalline structure at the same time, and this is how the state of aggregation known as supersolid is obtained. Very few predictions for the existence of supersolid materials have been published so far, and in particular in realistic and well-known materials such as deuterium," adds Dr. Hirschberg.
Machine learning makes friends with chemistry
The computational simulations conducted by the researchers were made possible by a new algorithm for describing special quantum systems developed by Dr. Hirschberg, during his stay as a postdoctoral student at the Federal Institute of Technology in Zurich (ETH Zurich). Thanks to this algorithm, based on a theory developed by the physicist Richard Feynman, Dr. Hirschberg was able to dramatically reduce the total number of calculations required to simulate the system. In addition, to describe the range of extreme pressures and temperatures, the researchers used tools from the field of machine learning, including an artificial neural network, to describe the interactions between the atoms in the material.
"The use of machine learning for the molecular forces is a promising direction in computational chemistry. The theoretical prediction we provide in the new paper opens up new horizons, because the relevant range of pressures and temperatures can be experimentally tested in the near future. Beyond that, we anticipate that the insights gained from the research will also be important in locating supersolids in other materials," concludes Dr. Hirschberg.
More of the topic in Hayadan:
- There is life in sinkholes
- Progress towards a quantum state of aggregation: a supersolid
- A glimpse into a new state of matter
- What do liquid crystals and wrinkle formation have in common?
- Researchers from the Hebrew University and Switzerland have developed a new experimental method for characterizing liquids found in the depths of the earth
Dr. Barak Hirschberg
