Through "evolution in a test tube" scientists of the Weizmann Institute of Science have created a molecule that may be used as an effective cure for Corona
Until a few weeks ago, it seemed that vaccines marked the way out of the epidemic for the world, but the constant appearance of new variants sharpens the understanding that along with vaccines there is also an urgent need for effective medical treatment against the corona virus. BPublished research in the scientific journal Nature Microbiology The scientists of the Weizmann Institute of Science - in collaboration with the Pasteur Institute in France and the National Institutes of Health (NIH) in the United States - developed a new therapeutic approach: instead of focusing on the "crown protein" of the corona virus responsible for the entry of the virus into the body's cells, the researchers turned the spotlight inward - on the protein located on our cell membranes and is the entrance gate of the virus. Using advanced methods of "evolution in vitro", the researchers developed a small protein molecule that acts as a "stopper" that blocks the entry of the virus into the body's cells, and may become a cure for Corona.
A significant part of the corona treatments that are currently in development - and the vaccines that are already in use - focus on the "spike" protein - that infamous protein that is on the envelope of the virus and gives it its famous crown shape. However, it is now known that this protein tends to develop mutations that erode the effectiveness of the various treatments. "Since the virus is constantly changing, we decided it would be more correct to focus on a protein that does not change - the ACE2 receptor through which the virus enters the cells of our body," explains Prof. Gideon Schreiber From the Department of Biomolecular Sciences at the Weizmann Institute. This therapeutic approach is expected to be more resistant to the appearance of new variants of the virus - the main challenge currently facing the eradication of the epidemic.
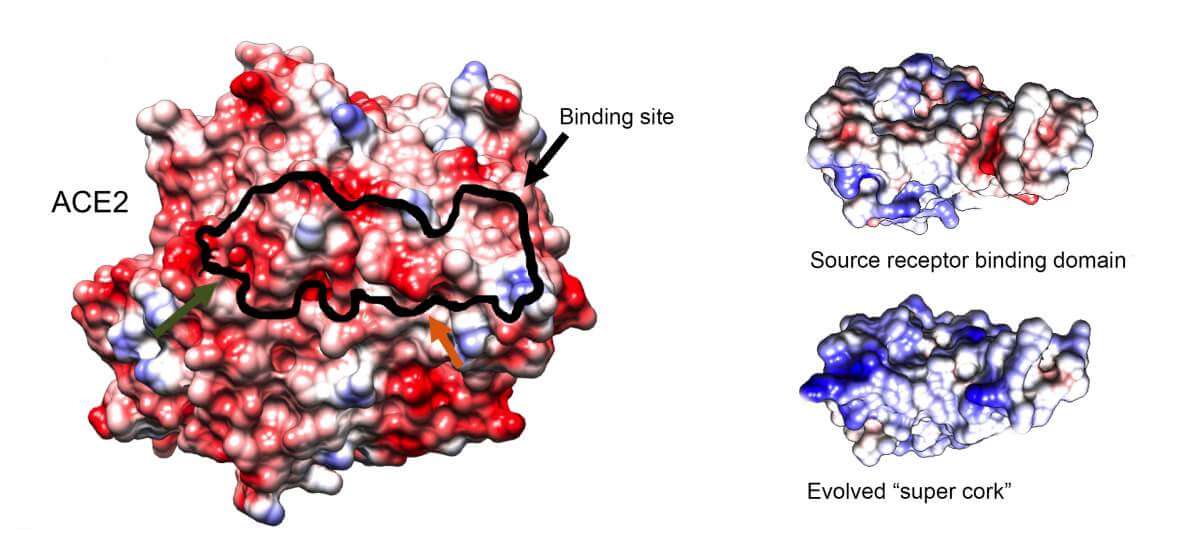
The ACE2 receptor, which is found on cell membranes that line the lung walls (hence the known damage of the corona to this organ) and other organs, is an essential enzyme that participates in blood pressure control processes. Therefore, as tempting as it may be, it is not recommended to develop a drug that will block its activity immediately. Accordingly, Prof. Schreiber's laboratory, which specializes in the study of interactions between proteins, aims to develop a protein molecule that will bind to ACE2 in a better and more efficient way than the viral crown protein - thus blocking the way of the virus from penetrating the cells - without disrupting the vital activity of the receptor
While scanning the millions of mutations received, the researchers discovered an amazing thing: already after the first evolutionary round, mutations were created in the laboratory that predicted the development of more infectious variants of the corona virus - the British (Alpha), the South African (Beta) and the Brazilian (Gamma) strains.
The scientists, led by post-doctoral researcher Dr. Yiray Zahardnik, isolated the ACE2 binding site on the crown protein - and put it through a series of rounds of "evolution in vitro". This method, which was perfected by Dr. Yeray Zahardnik, and simulates the processes of natural selection only at a much faster speed, makes use of a genetically modified strain of baking yeast. Because yeast cells are a convenient substrate for genetic modifications, the researchers could efficiently and quickly screen for millions of mutations created in the yeast cells during the rounds of artificial selection. "The ultimate goal was to find a molecule that would be significantly more 'sticky' than the original viral version and thus block the entry gate," says Prof. Schreiber.
While scanning the millions of mutations received, the researchers discovered an amazing thing: already after the first evolutionary round, mutations were created in the laboratory that predicted the development of more infectious variants of the corona virus - the British (Alpha), the South African (Beta) and the Brazilian (Gamma) strains. This fact clearly indicates the relationship between the strength of the binding to the ACE2 receptor and the degree of effectiveness of the virus. It is interesting that the mutations that characterize the Indian strain (Delta), the dominant strain today in many parts of the world, did not appear in the experiment in the yeast cells, since the mutations that made Delta so infectious primarily improved its ability to escape the immune system - and not its ability to bind to ACE2.
Among the millions of mutations created, the researchers isolated a small protein molecule whose ability to bind to the ACE2 receptor is 1,000 times higher than the binding ability of the original binding site of the virus. In addition, the research students Maya Shemesh and Shir Marciano showed that not only does this protein fit ACE2 like a bottle cap, it also does not harm the vital function of ACE2 at all. Moreover, thanks to its powerful binding capacity, extremely low concentrations of it were required to "cork" the entrance gate of the virus and block its path. Moreover, as the scientists predicted, the cork worked equally effectively against all known variants of the virus.
In order to turn the cork molecule into a potential cure for Corona, he teamed up in Prof. Schreiber's laboratory with Prof. Yanon Rodich from the Department of Earth and Planetary Sciences at the institute, and developed - in collaboration with Dr. Ira Merton and Dr. Chionlin Lee - a method for administering the protein to patients using a respiratory spray. This new therapeutic approach was successfully tested in an experiment in hamsters at the NIH - the treatment significantly reduced the onset of symptoms and their intensity. Additional preclinical studies are expected to take place at the NIH in the near future.
