The carbon atoms that are not directly connected to each other repel the other atoms and this force prevents the diamond from shrinking, explains Prof. Shmarihu Hoz, who headed the research group in a conversation with the Hidan site. The article is published in a journal dedicated to research in physical chemistry and according to Hoz it will provide tools for the development of even stronger materials * The team also includes Prof. Eli Altus from the Technion
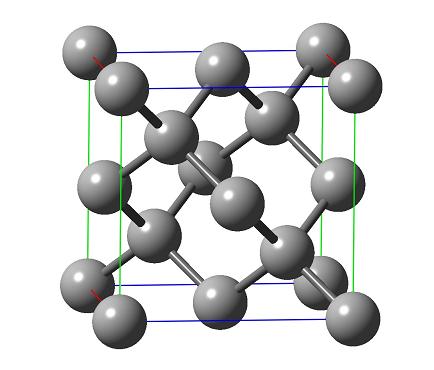
The team of researchers, led by Prof. Shmariahu Hoz from the Chemistry Department at Bar Ilan University, discovered that the interactions between the atoms in the diamond, which are not directly related to each other, are the main cause of the difficulty. "As we know, a diamond is composed of pure carbon," explains Prof. Huz. "Each carbon atom in a diamond is surrounded by four additional carbon atoms directly bonded to it, and each of these atoms is bonded to three additional carbon atoms. This is how a special structure is created in the composition of the diamond in which, around each atom there are 12 carbon atoms that are not related to it. In the research we discovered that the repulsion between these atoms is one of the main reasons why the diamond is so hard."
This discovery, which will be published in the next issue of the scientific journal The Journal of Physical Chemistry, could lead to the development of additional materials that will excel at a particularly high degree of difficulty, and perhaps even higher than that of diamond.
This is not the first time that this team of scientists has discovered important phenomena in the study of materials. The research group, which as mentioned includes Prof. Shmariahu Hoz, Prof. Harold Bash and Dr. Lior Itzhaki from the Bar-Ilan Chemistry Department, and Prof. Eli Altus from the Technion, has been engaged for several years in researching the validity of the laws of mechanical engineering in the nanometer world, and recently even revealed the existence of synthetic molecular rods whose strength is 40 times greater than that of a diamond. "The study of the nano world (whose units are measured in thousands of microns) using the tools of mechanical engineering, may be of great importance in expanding human knowledge as well as in the applied field." Prof. Hoz says.
In a conversation with the science website, Prof. Hoz explains: "Mechanical engineers use different tools to test materials. Using these tools it is possible to check the hardness of materials, their flexibility, when they break, etc. We tried to use these tools at the nanomolecular level and first started to examine whether rods, whose strongest structure as used in railway tracks and building beams, are also the strongest structure at the nanometric or molecular level. It turns out that this is not the case.
The strongest beam that engineers use is the so-called I BEAM, whose cross-section resembles the letter I, as seen in railroad tracks and beams that make up the skeleton of buildings. One of the questions we ask in our research is to what extent such a truth is also true in the nano world. "
"During the search we investigated different rods based on different materials and therefore also have different shapes. One of the rods we tested presented a strange phenomenon - when we stretched and compressed it, the "wrong" parts that we hadn't thought about bore the brunt of the contraction or stretching. When we looked carefully at these parts, we saw that what prevents the second unit from collapsing, shrinking or stretching (depending on the action we performed) these are the NON BONDED INTERACTION - interactions between atoms that are not directly related to each other."
What is meant by bonds between atoms that are not directly bonded?
Prof. Hoz: "When you look at a diamond, you see that it is made up of carbon atoms joined together. Carbon has four bonds and is therefore surrounded by four other carbon atoms to which it is bonded. Choose any one carbon atom and call it the central atom of the diamond. Of course, four other carbon atoms surround it in space. Each of them is connected to the central atom and three others, that is, in the vicinity of the central atom there are 12 more atoms (the 4 nearest neighbors to which it is connected and another 3 to which each of these neighbors is connected). These atoms are at a relatively small distance from the central atom and there is a repulsion between them and the central atom. When you press on an atom in a diamond, you decrease these distances and the repulsion increases. This is probably, we assume, the reason why diamond is so hard. There is another reason for the difficulty of the diamond and it is the classical reason. As we know, most matter (except for metals or noble gases) consists of separate molecules of matter. There are large gaps between the separates. When you press on the substance, what is compressed is not the molecules themselves, but the spaces between them are reduced. A diamond, compared to a normal substance, if it is perfect, is one big molecule and therefore difficult to compress, but there are still metals that are also one big molecule but they are not that strong. The reason for the excess hardness according to our proposal is those interactions between atoms that are not directly related to each other."
What makes these atoms repel each other and the "central" atom?
"By and large, this is the familiar repulsion between electron clouds: Inter-electronic Repulsion. Between any two molecules such as nitrogen molecules for example, the electrons repel each other and as a result the molecules bounce back to move away. As you try to bring them closer, the energy required for this increases. When the atoms are bonded together, the energetic gain of the bond offsets this repulsion and even more so, but when they are not bonded as in our case the 1 with the 12, it is a net repulsion effect."
How do you think the discovery will help to develop stronger products?

After publication, other scientists will try to verify the discovery, are you ready for that?
We came up with the idea, which is a kind of projection of a phenomenon known elsewhere in nature also on the diamond, and now it is the turn of the scientific community to investigate and verify or try to disprove the discovery. We move on anyway. Our innovation is in this modest donation. According to the professional literature in the field, this is the first time someone has come to such an insight and we hope it is true.

14 תגובות
The difference between difficulty and strength is substantial even though it sounds similar
Difficulty is a specific thing in a material that describes a small area where how it behaves is affected by the structure of the material
But strength is the strength of a model and it is also affected by the molecular structure but also by defects and cracks in the model, so it does not always depend on the difficulty
Regarding zirconium, as I know the structure of the atoms in it is not arranged like in diamond, that is, the arrangement of the atoms in it is close to a liquid and it is considered a liquid where the flow is very slow - glass is also considered a liquid.
Maybe durability!?!
------
Another little something about strength and difficulty
I remembered the statics classes (as part of architecture studies) and the beams and their ability to withstand carrying a load! If we transfer it to the world of diamonds, we can say that the diamond is strong under pressure like the family of stones, but weak (relatively) in bending, therefore a hammer blow will break it and a concrete cube will not!
Indeed, the "strength" of the concrete is tested by pressing a cube of a standard size (perhaps 10 x 10 cm) until it collapses and not by blows.. and therefore probably the correct term to use without confusing it is "durability" which means that the diamond is resistant to pressure and the steel is resistant to both, i.e. also to bending And for this reason, steel rods are inserted into the concrete to give it resistance to bending and thus it is possible to build on reinforced concrete beams (with steel or iron rods) without fear of bending and collapse and without the need to create arches as they did in the past (something that beautifies the buildings in my opinion). All the ugly housing estates in which the lower deciles were born, only a big rainbow will save them (and not just the eastern rainbow)!!!
An addition to Michael's words, it should be mentioned that the person who used this term was the interviewee, who believed that he knew what he was researching. I can have no discretion in such a case. (At least as long as it is not a translation of terms that have several options for translation but on a Hebrew basis - Bar Ilan's press release and the researcher's words in a special interview for the Hidaan website.)
Strength vs. Difficulty:
Before trying to understand the reasons for the "strength" of a certain material, one must zero in on the definition of "strength" that one wants to talk about.
Strength is a general term as it is easy to see also in the links provided by Brunel. The link itself is not to "strength" but to "compressive strength" and within it there is a link to "yield strength" (the description of which actually corresponds to the description of the "hardness" of a diamond).
Therefore, in my opinion, all the bickering I've seen here about the differences between strength and difficulty is inaccurate. According to the dictionary and also according to our intuitive understanding of the interpretation of the words "difficulty" is simply a type of "strength" and the comment about the inaccuracy in the article was out of place.
Since "strength" is a general quality, when talking about its causes, one must look for different reasons for each of its types.
N.C.:
Babylon's dictionary interprets the word "zircon" as follows:
"gem; mineral".
This means that, at least as far as the writers of this dictionary are concerned, the word "zircon" does not describe a particular material but refers to a family of materials.
Therefore, obviously, it is not possible to speak in general about the difficulty of these materials or about the differences between them and diamond.
This is what is called a diamond head!
And now (if we look from the opposite direction on Brunel's correct comment) it remains to find the internal reason for the strength of materials!!
By the way, is there a feverish formula in the world of functions? To check which functions and formulas are more powerful than others? Really!@!
What about zircons? Synthetic diamonds - what is their level of hardness compared to a natural diamond?
And how do they differ chemically from a diamond?
I would appreciate it if someone could enlighten me.
From Wikipedia:
hardness
strength
What a cap to him
I would be interested in getting the diamond from the experiment
If there were diamonds in vests, the IDF would go bankrupt, and many would go to war just to get such a vest...
Vests have Kevlar as far as I know...
Charles,
He is right. There is a difference between strength and difficulty. An everyday example of this is a crust of bread. The shell is hard, but easy to break. It is not strong, and does not stand up to stretching.
I've never heard of diamonds in vests. where did you see it
So why do they put diamonds in vests? If a hammer blow breaks them easily then what's the point?.. Maybe you're wrong?
The diamond, not strong, it breaks easily with a hammer blow,
The diamond is hard…..a mechanical property completely different from strength…