Weizmann Institute of Science scientists discovered that genes from the LATS family are tumor suppressors that constitute another line of defense of the body beyond p53. The findings may advance personalized breast cancer treatments

When someone exaggerates the importance of something marginal, it is customary to say that he "made an elephant fly". Weizmann Institute of Science scientists recently tested what happens when you "make a hippopotamus fly". The researchers focused on a biochemical reaction pathway known as the "Hippo pathway" - named after the hippopotamus, since disruptions in it cause fruit flies to grow to such large dimensions that they resemble those bulky mammals. Defects in this pathway in humans often lead to the development of cancer.
In fact, these are deficiencies in the activity of two genes in the "hypo pathway", LATS1 and LATS2, which are known as genes responsible for controlling the activity of the "guardian of the genome" in and of itself - the p53 gene. Dr. Noa Pirt and Dr. Yael Ilon became interested in these genes when they worked in Nablus in Nablus in the laboratory of Prof. Moshe Oren in the Department of Molecular Cell Biology, which specializes in p53 research. Dr. Elon is a faculty scientist in Prof. Oren's group, and Dr. Pirat was at the time a research student in the laboratory. The two wrote a research review on the role of LATS1 and LATS2 in cancer and discovered so much interest in these genes that Prof. Oren encouraged them to initiate and lead a study that would examine them in depth.
When the two set out, it was already known that the genes are expressed at lower than normal levels in malignant breast tumors. Dr. Pirt and Dr. Elon, together with their colleagues, planned a study designed to clarify how the low expression levels of LATS1 and LATS2 contribute to the development of this cancer, and more generally, how disruptions in the "hypo pathway" lead to cancer.
After a bioinformatic analysis of gene expression data in breast cancer, the scientists created two groups of mice with a tendency to cancer - one without the LATS1 gene and the other without the LATS2 gene. After the mice developed tumors in their mammary glands, the scientists compared gene expression in the tumors in each of the groups as well as in tumors with normal copies of the genes from the LATS family. In addition, in collaboration with researchers from the University of Athens in Greece, they examined gene expression in the tumors of women with breast cancer.
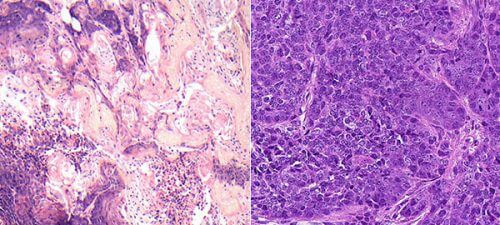
As recently reported in the scientific journal Life Science Alliance, the study confirmed that genes from the LATS family are tumor suppressors and that they prevent the cell from becoming cancerous. In other words, this is an additional line of defense beyond the p53 - similar to an additional brake system in a car, a manual brake added to a foot brake. This finding explains why cancer can break out even when p53 is working properly; Sometimes, the cause is a defect in the genes that "guard" p53 and not in the "guardian of the genome" itself.
The researchers also discovered that despite the family resemblance between LATS1 and LATS2, each of the genes plays a different role in protecting against breast cancer. When the activity of LATS1 is disrupted or completely lacking, tumors developed in mice with characteristics of basal breast cancer (basal-like); Tumors of this type are more aggressive and do not respond to hormonal therapy. In contrast, in the absence of LATS2, the tumors that developed had characteristics of luminal breast cancer. Luminal tumors are often dependent on the hormone estrogen, so it is sometimes possible to treat them with estrogen-blocking drugs, such as tamoxifen. "Breast cancer is a general term, it includes different types of cancerous tumors, each of which can actually be treated as a completely separate disease," explains Dr. Pirat.
The scientists identified additional genes, some in the "hypo pathway" and some outside it, which contribute to the proliferation of cancerous tumors characterized by the inactivity of genes from the LATS family. This is how they discovered that a low expression level of LATS2 leads to a metabolic change that increases the cell's dependence on glucose - the energy provider for the cancer tumor. When the scientists increased the activity of LATS2 through genetic engineering and exposed the cancer cells to rosiglitazone - an anti-diabetic drug that changes the body's glucose metabolism - this often led to the elimination of these malignant cells.
As the findings are expanded in further studies, they may promote personalized breast cancer treatments. For example, the decision regarding hormone therapy is currently based mainly on a test of estrogen receptors in the tumor cells. A genetic test that will determine whether the tumor is characterized by a low expression level of LATS1 or LATS2 may provide a more accurate criterion for the question of whether hormone therapy may help.
"Also, the discovery that tumors, in which the level of LATS2 activity is low, are dependent on glucose, may help to develop new treatments for tumors characterized by increased glucose metabolism," says Dr. Elon.
Dr. Ioannis Petras, Dr. Vasiliki Vlacho and Prof. Vassilis Gorgoulis from the University of Athens also participated in the study; Dr. Ron Rothkopf, Dr. Helena Einbinder and Dr. Dina Leshkowitz from the Department of Life Science Research Infrastructures of the Institute; Dr. Randy Johnson of the University of Texas MD Anderson Cancer Center; and Irina Rivkin, Ina Schmidt, Deborah Bekiev, Anat Gershoni and Prof. Moshe Oren from the department of molecular cell biology at the institute.
It is possible to sort the types of breast cancer into at least 4 groups according to the molecular characteristics of the tumor cells. New studies based on advanced genomic technologies expand the division into 11 subtypes.
- More of the topic in Hayadan:
- The genes are not only selfish, sometimes they work in groups * The discovery will help in a more successful diagnosis of breast cancer
- A method for detecting breast cancer based on quantum technologies
- Has a new cause of breast cancer been found? / Melinda Wenner Moyer
