How can the activity of the 3-GSK enzyme, whose overactivity leads to the appearance of diseases such as diabetes and Alzheimer's, be curbed? Using computer models produced by scientists from the Weizmann Institute, a research group at Tel Aviv University succeeded in developing a short protein ("peptide") that competes with the substrate to which the enzyme binds, thus inhibiting the activity of the enzyme. When the peptide was administered to mice suffering from a disease similar to Alzheimer's, it became clear that their symptoms were improved following its use.
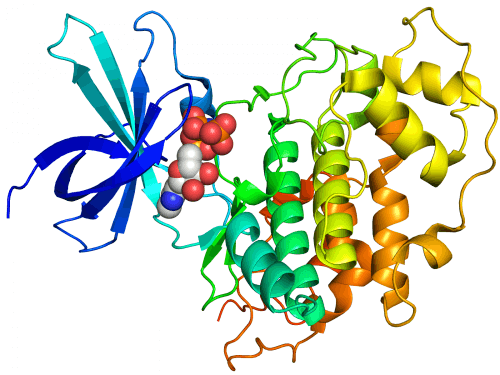
"Creating computerized molecular models is sometimes the only way to understand exactly how complex molecules such as proteins work together," says Dr. Miriam Eisenstein, head of the unit for building macromolecular models in the Department of Chemical Research Infrastructures at the Weizmann Institute of Science. Already about two decades ago, as part of a joint research she carried out with the late Prof. Ephraim Katzir, Dr. Eisenstein was among the first in the world to develop computerized methods for predicting how biological molecules connect to each other (Molecular docking methods). Since then, she has collaborated with a wide variety of research groups, inside and outside the institute.
Despite her extensive experience in the field, Dr. Eisenstein testifies that the behavior of proteins sometimes manages to surprise her. This happened, for example, in a study she recently conducted jointly with the research group of Prof. Hagit Alder-Finkelman from Tel Aviv University. the study Released Recently in Science Signalingg. "The findings of this study, from which a new and promising direction for the treatment of Alzheimer's emerged, were certainly surprising," she says.
The study dealt with an enzyme called 3-GSK, which affects the action of other proteins in the body, and is therefore very essential. "3-GSK adds a chemical tag (a phosphate group) to other proteins, substrates, and thus determines their fate - it determines whether they will be active, inactive, or destined for destruction," explains Dr. Eisenstein. "The source of phosphate is in a small molecule in the cell called ATP. This molecule, together with the substrate, binds to the enzyme, and the enzyme transfers a phosphate group from the ATP to the substrate." The problem is that in a state of overactivity, GSK-3 contributes to the appearance of various diseases, including diabetes and Alzheimer's. For this reason, Prof. Alder-Finkelman's laboratory was looking for a method that could curb its activity.
"Until now, scientists' efforts to curb the activity of GSK-3 have focused on blocking the binding of the enzyme to ATP. But the prevention of this binding is not selective," emphasizes Dr. Eisenstein, "which means that it leads to the blocking of other similar enzymes, and as a result, unwanted side effects may appear."

In light of this, Prof. Alder-Finkelman and her group decided on a different direction in their research. They sought to inhibit the activity of the enzyme not by means of ATP, but by means of that region in the enzyme to which the substrate binds. "The idea was to try to develop in the laboratory an alternative protein for the substrate, which could compete with it for binding to the enzyme, thus interfering with the activity of the enzyme," explains Dr. Eisenstein. The research group at Tel Aviv University performed a series of experiments in the laboratory, and managed to identify a peptide (a short sequence of amino acids, the building blocks of proteins) suitable for this purpose. At this point, Prof. Alder-Finkelman turned to Dr. Eisenstein with a request to help her improve the peptide, and to design additional peptides that would compete with the substrate more effectively. "I got involved in the research, and in the first step I created computer models of the binding of the enzyme with the substrate, and of the binding of the enzyme with the peptide," says Dr. Eisenstein. "In light of what the models showed, I suggested to Prof. Alder-Finkelman's research group to make some improvements in the structure of the peptide, and in the laboratory experiments it performed it became clear that these improvements are indeed beneficial. In doing so, they supported the correctness of the computer models."
Later, the scientists from Tel Aviv University jointly designed some additional peptides with Dr. Eisenstein, and tested their ability to bind to the enzyme. "Based on my calculations, I predicted that one of those peptides would function as a substrate, and in laboratory experiments it turned out to be the case. That is, in the laboratory it turned out that the peptide binds to the enzyme and receives a phosphate tag," says Dr. Eisenstein.
But then the scientists realized that at the end of the labeling process the peptide remained bound to the enzyme. "This binding was more durable than the binding of the original peptide," says Dr. Eisenstein. "This was a surprising finding, since after labeling, the substrate was supposed to be rejected from its binding site by the enzyme and separated from it." The calculations she made clarified the picture. "We discovered that the tagged peptide changes its shape, and binds to the enzyme in a different way compared to the initial binding as a substrate. The result is a particularly strong binding: this is how the tagged peptide prevents the enzyme from binding and tagging other substrates."
Prof. Alder-Finkelman's research group tested the effectiveness of the peptide in animal experiments. They gave the peptide to mice suffering from a disease similar to Alzheimer's, and found that the mice's symptoms were improved following its use. "The success of the experiment illustrates that there is great value in collaborations between biologists and scientists dealing with computerized molecular models," says Dr. Eisenstein. "Prof. Alder-Finkelman deeply understands the activity of GSK-3 and the need to control its activity, while I am interested in the way proteins bind and interact, and bring structural insights to the research."
In conclusion, she adds: "Building computerized molecular models is a very useful scientific tool, which gives me the opportunity to collaborate with diverse research groups and take part in many interesting studies. Although, over the years, the computers I work with have become more powerful and sophisticated, but the basics of chemistry remain the same, and our work based on the combination of the two is more relevant than ever."
