Researchers from UCLA demonstrated a method for isolating two different molecules together using a substrate and controlling their reaction when exposed to ultraviolet radiation
Good chemists have passive-aggressive behavior - they influence molecules without actually touching them. In an attempt to control substances on a nanometer scale, researchers from UCLA demonstrated a method for isolating two different molecules together using a substrate and controlling their reaction when exposed to ultraviolet radiation while receiving detailed observations before and after the reaction. The research findings were published in the scientific journal Science.
"This is one step in measuring and understanding the interactions between light and molecules, which we hope will eventually lead to more efficient conversion of sunlight into electrical energy or other useful forms of energy," said lead researcher Paul S. Weiss, professor of chemistry and biochemistry at UCLA. . "In our work, we used energy derived from light in order to initiate a chemical reaction that would not have occurred if the molecules were free to move in the solution; They were held in place by their anchoring to the substrate and by the inactive medium of the molecules surrounding them."
The control of the exact way in which molecules join together in order to study the reactions that take place is known as regioselectivity Regioselectivity is extremely important in chemical synthesis, because it allows the receipt of a specific and known main product, without side reactions that compromise efficiency and utilization. One of the ways to direct a reaction to obtain one defined product is to isolate the molecules and hold them together to obtain regioselective reactions; This is the strategy that enzymes use in their activity in many biochemical reactions.
"The special scanning tunneling microscope that we used in these studies can also be used to measure the absorption of light and measure the charge separation in the molecules used in solar cells," explains the lead researcher. "This method provides us with an innovative way to optimize the activity of these molecules, while collaborating with researchers in the field of synthetic chemistry."
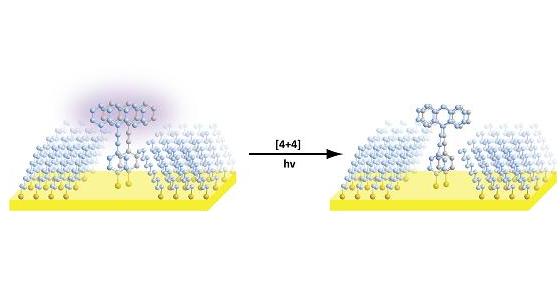
The researchers were able to isolate and control the reactions of a pair of molecules by constructing specially prepared nanostructures to force only the two molecules to stay in place. The molecules used in the research are sensitive to light and are used in organic solar cells; Similar methods can be used in a diverse and extensive study of molecules. Controlling how the molecules in organic solar cells join together may ultimately lead to increased cell efficiency. In order to isolate the two molecules and orient them in the desired - and unusual - way, the researchers used an idea similar to that found in baby toys where they have to match shapes to their appropriate places.
The researcher created a defect, or hollow niche, in a self-assembled monolayer (SAM) - a single layer of molecules anchored by a smooth surface - in this case, gold. The size and shape of the defect in the monolayer were designed in such a way that only two defined organic reactant molecules would adapt to it and build up with each other in the desired configuration exclusively. In order to guide the grouping of the molecules into the monolayer in the desired direction, a sulfur atom is attached to the bottom of the molecules since this atom is easily attached to a gold surface.
"The usual process for this type of chemistry is characterized by mixing a collection of molecules in a solution and waiting for them to form with each other, but in an almost random manner, so that only three percent of the molecules may react in this way," explains the researcher. "Our method is much more targeted and efficient. Instead of performing one measurement on thousands of molecules, we perform a multitude of measurements on only two molecules."
After the molecules were isolated and anchored to the surface, they still had to be excited by radiation to initiate the reaction. In this case the energy was provided by ultraviolet radiation which initiated the start of the reaction. The researchers were able to verify the proper arrangement and the course of the reaction of the molecules using the special microscope developed in their laboratory.

One response
Well done for the article.