A review of some of the most promising medical devices currently in development
In recent years, researchers have taken advantage of the breakthroughs in human biology, electronics, and genetics to develop a new and impressive set of tools to improve and maintain human health. Sophisticated medical technologies and complex data analysis methods are currently on the verge of breaking through from their traditional fields in hospitals and computer labs, towards their use in our everyday lives.
Doctors of the future will be able to use these tools to track patients and predict how they will respond to personalized treatment plans based on their unique physiology, rather than guessing based on the average response rates of large population groups as is common in clinical trials today. Innovations in the miniaturization of computer chips, bioengineering and materials engineering lay the foundations for new devices that can replace complex organs such as the eye or the pancreas, or at least help them function better.
The articles on the following pages offer a glimpse at some of the most promising developments in personalized technology in the fields of genetics, artificial vision, cancer, implantable monitoring devices, and mental illness. Not all developments will necessarily succeed. But as a whole they imply that the role of tiny medical technology will increase, not only in treating the sick but also in maintaining the health of the healthy.
Genetics
Personalized medicine
The price of sequencing a person's genes continues to drop, but understanding the meaning of the sequence continues to be a challenge
When the Human Genome Project was launched more than twenty years ago, everyone expected that printing the complete instruction book for creating a human would require hundreds of devices to determine the DNA sequence, cost three billion dollars and take 15 years. 13 years later, in 2003, the first "complete" human genome sequence was announced. However, this important achievement was still a work in progress. Huge gaps that have not yet been completed then remain in the map of the hereditary material that determines a person's genetic destiny.
Fast forward to January 2012, at the International Consumer Electronics Show in Las Vegas, there was a gene sequencing device between computer game consoles and flat screen televisions. It was a bright white box about the size of a desktop printer. The device's inventors claim that when it hits the market later in 2012, it will determine a person's entire genetic sequence in just a few hours for $1,000, that is, the price of a plasma television.
For years, a $1,000 genome has been touted as the tipping point that will usher in a new era of personalized medicine. At this price, the expense should be cheap enough for doctors to work with such devices to treat patients with heart disease, cancer or other diseases. This treatment should be based on the patients' personal genetic risks and their individual drug sensitivities. According to experts who follow the development of the industry, the more devices for determining the genome sequence, such as the one presented at the exhibition, become available, the sooner the era of comprehensive genetic testing of the population will begin.
However, some argue that the time has not yet come for extensive public disclosure of the technology. "She's not ready," says Aravinda Chakraborty, a professor of genetics at the Johns Hopkins University School of Medicine. Chakraborty is concerned that the potential benefits of personalized genomic medicine have been overstated. People don't realize, he says, that comprehensive genetic scans performed by a doctor or bought online are almost worthless as medical tools today.
The main problem is that the technology has developed faster than the researchers' ability to understand the results it produces. For example, to understand which genetic patterns are markers of disease and which patterns can be safely ignored, doctors must compare each individual's genetic result with many other people's results. Also, many diseases are caused by rare mutations that have not yet been identified.
Finally, the task of sorting through the vast amount of information that emerges from a genomic scan is a daunting one. "The production of information today is fast and cheap," says Ewan A. Ashley, junior professor of cardiology at Stanford University School of Medicine. "But analyzing the results? Wow. It's not going to be quick, and it's not going to be cheap."
To demonstrate how complex the process can be, Ashley and several researchers at Stanford and Harvard universities analyzed the genome of their colleague Stefan Kwik, a professor of bioengineering. It took them six months just to figure out how to do this, even though Quick had already sequenced his own genome, so they had the raw data.
Quick's family history included several cases of heart disease. It is understood that the team found that he had several genetic variants associated with an increased tendency to heart attack. However, the genetic analysis revealed some unexpected results, including a greater likelihood of a hereditary blood disorder called hemochromatosis, although no one in Quick's family suffers from this disorder. At this stage it is impossible to determine whether the unexpected result reflects a real danger or some error in the process of determining the sequence, the genetic equivalent of a typographical error.
Despite these issues, Ashley is optimistic about the potential for personalized DNA results to transform medical care. He foresees days when the human genome will be an integral part of electronic medical records. However, so far, the few patients who have benefited most from having large sections of their genomes analyzed have had rare diseases with genetic variants that stood out for being unusual. For most of us, our genome is still shrouded in fog - an untold legend.
Nancy Schutt
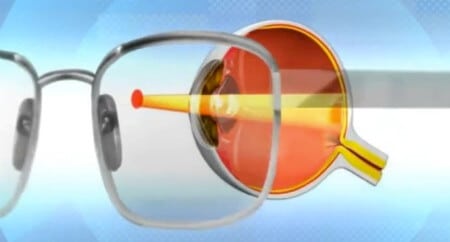
artificial vision
bionic eye
Artificial light receptors will restore sight to the blind
Mika Terhu knows the difference between an apple and a banana. He can tell you that one is round and sweet and crunches when you bite into it, and the other is long and curved and turns to mush if it cooks too long. But if you ask him to differentiate between the fruits without touching, without smelling or without tasting, he will be lost. Trejo is completely blind. However, for three months in 2008, he regained his ability to distinguish between an apple and a banana with the help of vision thanks to a tiny electronic chip implanted in his left eye. Although short-lived, the initial success of the new technology permanently changed the prospects of Treho and many others like him.
Treho, who works at a sports scholarship organization in Finland, has leuka retinitis pigmentosa, a genetic defect that destroys the light-sensitive cells in the retinal wall at the back of the eye. He saw perfectly well until the age of 16, then his night vision began to suffer. In his 20s, his ability to see during the day also deteriorated. At the age of 35, Treho lost the central vision in both eyes, and when he was 40, he was able to perceive only fractions of light in his peripheral vision.
In November 2008, everything changed, when Eberhart Zerner of the University of Tübingen in Germany implanted the chip in Trejo's retina. The chip replaced damaged light receptors (called cones and photoreceptors) in the retina. In a healthy retina, the receptors convert light into electrical impulses that eventually reach the brain as they travel through several layers of specialized tissue. One of the layers consists of cells called bipolar. The chip contains 1,500 squares arranged in a grid measuring 0.3x0.3 centimeters, and each of them contains a photodiode, an amplifier and an electrode. When light hits one of the photodiodes, it generates a tiny electric current that is amplified by a nearby amplifier. The electrical current is delivered to the electrode and it in turn excites the nearest bipolar cell which eventually sends a signal through the optic nerve to the brain. The more light hits the photodiode, the stronger the electric current produced as a result.
Treho's retinal implant opened a window to a world the size of a piece of paper with an area of about 50 square centimeters standing at arm's length from his eyes. Through this window, Trejo could identify basic shapes and outlines of people and objects, especially if there was a sharp contrast between light and dark colors. However, the implant did not contain enough electrodes to produce sharp images. Also, the chip allowed Tarho to detect only shades of gray because the chip does not differentiate between different wavelengths of light.
Despite these limitations, within days of the surgery, the implant had significantly changed the way Trejo interacted with the world. For the first time in a decade, he was able to see and name items such as silverware and fruit, read large print, approach people in a room and recognize loved ones. Two other patients who underwent transplantation at about the same time were able to detect bright objects placed against dark backgrounds.
Zerner had to remove the chips from his patients after three months because, following implantation, they were prone to skin inflammation: a small external battery transmitted electricity to amplifiers inside the eye through a cable threaded through the skin, leaving an open wound. Furthermore, the users had to be near a computer that wirelessly controlled the frequency of the electrical impulses and aspects of vision such as brightness and contrast.
Since 2008, Zarner has made his implant safer and more portable. The latest model is wireless, and has been implanted in ten people so far. A thin cable passes under the skin a short distance, and connects an electromagnetic coil located behind the ear with a chip located behind the eye. By placing another electromagnetic coil, which is housed in a small plastic box above the skin next to the ear, an electrical circuit is closed that supplies electricity to the implant. Patients can change the brightness and contrast using buttons on the outer coil. To add to and improve the technology, Zarner wants to implant three chips next to each other in one retina, so that people have a wider field of vision.
Although synthetic photoreceptors may be helpful in any form of blindness resulting from damaged photoreceptors (ie, retinitis pigmentosa, choroideremia, and various types of macular degeneration such as geographic atrophy), they cannot help people with glaucoma, cataracts, or disorders where the optic nerve degenerates.
Another team reached Zarner's level of success in clinical tests. The Second Sight company from California has developed an implant designed for the retina, its name Argos II, which also treats retinitis pigmentosa, albeit with a different approach. Argos II captures images of the world with a tiny camera mounted on a pair of glasses, converts these images into electrical impulses and transmits them to an electrode that is placed on the surface of the retina and is not implanted inside it. Unlike Zerner's implant, Argos II does not mimic normal retinal excitation using light waves, but instead creates a mixture of bright and dark spots that patients must learn to interpret.
Even restoration of grayscale vision is expensive. Today, the price of the Argos II array is $100,000 per eye, as is the price of Zerner's retinal implants, after a comprehensive examination and approval. Zarner must perform additional clinical tests before European advisory boards approve him to teach other surgeons how to perform the procedure. Argos II has been approved for sale in most European countries, but has not yet been approved in the US. However, given the success of the first clinical trials and the speed at which the technology is improving, it appears that retinal implants may become more widely available in just a few years. [A company operates in this field in Israel Nano retina – the editors]
Paris Jaber
Early detection
to get over the cancer
Bioengineers are developing tiny nanoparticles programmed to detect cancer in its earliest stages
Tiny particles have the potential to solve one of medicine's biggest problems. Nanoparticles, whose size is measured on the scale of nanometers (billionths of a meter), are so tiny that 500 of them can be placed across the width of a human hair. Scientists aim to engineer them to perform many actions, from delivering drugs between certain organs in the body to creating detailed images of internal organs. Researchers are currently targeting them for the purposes of detecting cancer cells hiding in their hiding places.
Today, tumors can only be detected after they have reached a size that allows the imaging devices to see them during the scan. Nanoparticles, on the other hand, can detect a single cancer cell in a sample of 100 million normal cells. For example, in the experimental detection of breast cancer using nanomedicine, the researchers discovered tumors 100 times smaller than those that can be seen in a laboratory mammogram. Nanoparticles linked to proteins unique to cancer or genetic material can also help doctors distinguish between malignant tumors and normal inflammation or benign lesions.
Gregory Lanza, a professor of biomedical engineering at Washington University in St. Louis, and his team are developing nanoparticles that look for and signal the presence of new blood vessels that are characteristic of the main developmental stage of the development of cancerous tumors, such as colon cancer, breast cancer and other tumors. The development of such blood vessels does not normally occur in non-cancerous tissue. Theoretically, this technology can also inform doctors about the speed of cancer development, and accordingly it is possible to determine how aggressive the treatment should be.
Sanjeev Sam Gambhir, a professor of diagnostic radiology at Stanford University, and his colleagues are focusing on colon cancer and trying to find tiny malignant tumors that a regular colonoscopy might miss. The team creates nanoparticles made of gold and silica, on which molecules are added that guide the particle to home in on certain cancer cells. When the targeted molecules bind to a tumor in the colon or rectum, the minerals that make up the nanoparticle scatter the light coming from a special endoscope and reveal the presence of the cancer.
Nano-engineers are also trying to build nanoparticles that perform a variety of tasks, such as highlighting tumors in scans such as MRI, PET and others, and even directing drugs towards the cancerous tumor. Such integrated nanodevices may allow doctors to see if a treatment has reached its target and if it is actually working. Doctors are often unable to determine to what extent the drugs have actually reached the tumor, even with the help of today's targeted therapies, which work uniquely on cancer cells and skip normal cells. "The imaging component is what makes it possible to know that the drug was actually transferred, and to what extent," says Lanza.
Efforts to bring the use of nanoparticles to the clinic encounter several obstacles. For example, researchers will have to prove that these tiny tools are safe for use in humans. But the "biggest obstacle" to cancer treatment, says Gambhir, is the lack of possible targets. Nanoparticles can be designed wonderfully, but they are "not magic," he says. The researchers do not have enough knowledge about the earliest stages of cancer development, with the help of which it would be possible to know which molecules to direct the nanoparticles towards. Without knowledge of the goals, "we haven't even taken the first step yet," says Lanza. "We have to walk before we can run." As a variety of industry analysts estimate that the global nanomedicine field will surpass $130 billion by 2016, the race for discovery continues.
– Katrin Harmon
Remote measurement of personal data
Smart implants
New wireless monitoring devices warn patients of an impending heart attack or help them control diabetes
Biomedical engineers are developing tiny implantable monitoring devices that will help reduce the amount of guesswork in determining the best treatment for chronically ill patients, such as heart disease or diabetes. Some such devices, currently being tested in clinics, send information wirelessly from central areas of the body or blood vessels to external receivers. Ultimately, implantable monitoring devices may take a more active role in the treatment itself. For example, they will not only detect dangerous heart rhythm disturbances, but will also be able to revive a heart that has died. Several devices in the development stages are designed to deal with two of the most common medical problems:
Heart attacks. The AngelMed Guardian device is manufactured by Angel Medical Systems in Shrewsbury, New Jersey. It is about the size of a pacemaker and it monitors the heart, beat by beat to detect abnormal patterns, such as a sharp increase in the timing of heartbeats or an irregular heartbeat. The device is used by people who have recently had a heart attack (and are therefore at risk of another heart attack), and they do not meet the requirements for a pacemaker or a defibrillator implant. If the device senses that a heart attack is about to occur, it vibrates and causes an external detector to beep and flash, thus alerting and warning the patient or those around him and urging them to seek help. To avoid false alarms, the device should detect a problematic signal for more than a minute before sending an alert. For data analysis, the information collected by the device can be wirelessly downloaded to a computer. Angel Medical Systems has given a company that manufactures implantable defibrillators a license to use its technology to monitor heart rhythm. The integrated technology will allow the defibrillator to deliver an electric current to the heart if the monitoring device picks up signs of cardiac arrest or a particularly dangerous disturbance in the heart rhythm, and at the same time the results of the electrocardiogram will be sent from the device to the doctor.
Abnormal glucose levels. A new implantable glucose sensor manufactured by GlySens in San Diego may one day offer millions of diabetics their own wireless monitoring system. The device is implanted under the skin, and almost continuously reads the glucose levels in the subcutaneous area and they are then adjusted to the blood glucose levels. The result: accurate and comprehensive information, with the help of which the dose and timing of insulin administration can be determined, much more than the information that can be obtained from blood tests obtained by finger pricking. Since the sensor is implanted, it also requires less maintenance compared to external monitors available today.
"We are interested in giving the patient and his family a device with which they can actually forget about its existence and just receive the information," says Joseph Lucisano, a bioengineer who is also the president and CEO of GlySens. According to him "Treatment of diabetes and many other chronic diseases is based on monitoring, identifying and optimizing signaling patterns." So a wireless link that transmits "large volumes of information at a minimal price, will allow many things that we probably are not even able to anticipate."
Wireless sensors are expected to be less prominent in the future. Researchers have developed a thin and flexible device capable of collecting heart rate readings, muscle contractions and even brain waves that can be implanted as a temporary tattoo on the skin or inside the body. The innovative electrical circuit was developed by the mc10 company from Cambridge, Massachusetts, which manufactures flexible electrical components. This device will become completely portable, with an internal power supply and a wireless transmitter. It is quite likely that the combination of wireless monitoring of internal organs with flexible technology attached to the organ will soon provide patients and doctors with immediate information about a wide variety of chronic diseases, which for a long time have been difficult to control.
– Katrin Harmon
Brain science
Blood tests for mental illness
Levels of certain proteins may offer a new way to diagnose schizophrenia and depression
Sabin Ban seeks to change the way psychiatrists diagnose serious mental illnesses. Over the past 15 years, she has rummaged through the blood and brains of patients with schizophrenia and bipolar disorder (in which the person's mood swings between mania and depression), in search of proteins that indicate the likelihood that a person will develop these diseases. The molecules, known as biomarkers, promise an objective way to identify mental illnesses much better than the conventional approach of diagnoses based mostly on the patient's self-reported behavior patterns.
Although the biological markers have improved diagnostic methods for many diseases, including diabetes and heart disease, they have not been effective so far in diagnosing mental illnesses. And yet, Bunn, who runs a laboratory at the University of Cambridge, and several other neuroscientists, are convinced that biomarkers will soon become a necessary component of the tools available to psychiatrists. Two blood tests are already commercially available. One of them is based on Ban's research.
In 1997, Bunn began scanning the brains of dead men and women. She found that compared to the brains of healthy people, the levels of at least 50 proteins in the samples she tested were unusually high or low. Of these, 19 proteins were involved in the activation of mitochondria, the tiny organelles responsible for energy production in cells. Ban also found evidence that the neurons of schizophrenic people are unable to utilize glucose efficiently, and rely on another molecule, lactate, as an alternative energy source.
By 2006, Ban had found similar biochemical differences in the cerebrospinal fluid and blood of schizophrenic people. In two of her most recent studies, in which she tested the levels of 51 proteins in the blood, she was able to distinguish between schizophrenic patients and healthy people with an accuracy of about 80%. This group of biomarkers includes the stress hormone cortisol and the protein BDNF (brain-derived neurotrophic factor) which encourages the growth of new nerve cells and creates new connections between existing nerve cells.
Based on Bunn's research, Myriad RBM Labs in Austin, Texas, developed a blood test for schizophrenia called VeriPsych that costs $2,500. This test measures the amounts of different proteins identified by Ban. Although the US Food and Drug Administration (FDA) has not approved the test, psychiatrists are allowed to use it as part of their training. (Some tests limited to a single laboratory do not require approval from the FDA as long as they meet standards for human use.)
Similarly, San Diego-based Ridge Diagnostics has developed a biomarker test for depression. The company provides the test through a laboratory in North Carolina for $745. The test, called MDDScore (an index of clinical depression), looks for 10 biomarkers in the blood, including BDNF and cortisol.
Researchers have not yet validated these blood tests in clinical trials, except for small studies funded by the companies themselves. However, some psychiatrists have found the tools helpful in distinguishing between schizophrenia and drug-induced temporary psychosis. Also, these tools help depressed patients come to terms with their illness and receive treatment.
– Paris Jaber
___________________________________________________And more on the subject

3 תגובות
There is an Israeli company whose research results in the fields of treating psychological disorders should definitely have earned it an honorable place in this article. The company already markets equipment for the treatment of depression in the European market in Israel and other countries. Attached is a link to download the company's presentation for those interested.
http://maya.tase.co.il/bursa/report.asp?report_cd=730030
Asaf about personalized medicine, I completely agree with you.
I'm not an expert in the field, but here are some of my thoughts about the article:
Personalized medicine - I don't buy the fact that it is impossible to understand the genome. If there is a good method to read
The genome will very quickly accumulate enough readings for simple computer programs to start finding patterns and the research can even pass to the individual as is done in various distributed computing projects.
A bionic eye - according to several decades, they managed to experiment with cats, so for some reason it is still not practical, so it is not as simple as it sounds.
Early detection - a recent experiment found that women who underwent periodic mammography have a greater risk of cancer than women who were not tested. The conclusion is that probably some of the small tumors disappear on their own without the need for treatment. But if we discover them at the very beginning of their formation, there may be an unnecessary intervention.
Smart implants/heart attacks - there is no 100% error-free device. What happens if such a device detects a cardiac arrest even though there really isn't a problem?
Abnormal glucose levels - there are already monitors today, it would be nice if they were wireless, but this is not a fundamental change in the treatment offered today.