Along with the increasing interest in the use of nanoparticles for a variety of fields, starting with antibacterial substances, for medical imaging and ending with electronic devices, the need to understand the environmental, health and safety risks of these particles also increases. Researchers from the National Institute of Technology and Standards (NIST) have developed a simple process for producing nanocrystals that will allow studies to be conducted regarding certain chemical and physical properties responsible for the activity of these nanoparticles with their surroundings.
Since nanoparticles behave differently than bulk forms of the same material, new tests must be developed to examine their effect on biological systems. Toxicologists (poison experts) determine the dangers arising from nanoparticles by introducing them into biological systems and monitoring their effects on them, but they currently lack a series of reference particles whose size, shape and composition have been prepared and determined as required.
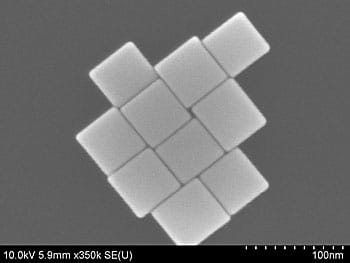
In a new paper, published in the scientific journal Angewandte Chemie, scientists from the institute describe a one-step process that allows them to control the size, shape and composition of gold/copper alloy nanocrystals while obtaining a nanocube with perfect wigs measuring 3.4 nanometers - about half the thickness of biological cell membrane and in the same order of magnitude as DNA.
The researchers mixed and heated starting materials for gold and copper with other chemicals to obtain perfect, uniform and crystalline nanocubes, with high utilization. In order to test the production process, they took samples after one hour, one and a half hours, five hours and twenty-four hours and discovered that only five hours are enough to make perfectly cube-shaped nanoparticles of uniform size. By adjusting the relative amounts of the chemical starting materials and the reaction time, they were able to precisely control the size, shape and composition of the nanocubes. The process is unique in that it allows control of the ratio of the gold atoms compared to the copper atoms inside the nanocube so that it is 3:1 or 1:3.
"This is a simple process, and to the best of our knowledge, it is the first ever to use synthetic chemistry in a "bottom-up" approach to the production of a gold nanocube with a size that is below five nanometers. Any preparation of a structure smaller than ten nanometers was challenging due to the changing behavior of the gold atoms," says physicist R. Hight Walker, who was one of the authors of the paper.
The innovative process for preparing such nanocubes will allow toxicologists to systematically change one of the properties of the nanocube at a time and measure the effect of this change on the biological response, if any.
This synthesis, and the high-quality nanocubes obtained through it, could be used in other applications in fields such as solar energy, says the researcher. "Normally, we are not able to prepare large quantities of high quality samples for experiments; Now we are able to."
The perfected nanocubes differ from other nanocubes known in the scientific literature, the researcher notes. The sharp tips, as opposed to truncated or rounded tips, will allow for a different, more active chemistry, which can benefit applications such as catalysts - through which the nanocubes can be used to initiate or accelerate chemical reactions.
