Will a light and fast particle located in a container inside which a heavy piston operates, exchange energy with the piston and reach an energy equilibrium with it, on average?

Haste, as the Arab proverb says, is from the devil. On the other hand, "faster, higher" is one of the slogans that best describes the human drive for achievement. In many complex systems, from the system of human culture to defined and closed physical systems, components that move fast and other components that move slowly exist and mix with each other. So, for example, when in one container there are light particles that move fast and heavy and slow particles, we tend to think that they will collide with each other again and again, so that the fast particles and the slow particles will transfer energy to each other, until, on average, the energy of all the particles will be equal, and the system will reach equilibrium - Energy weight. This is the basic assumption in the field of statistical mechanics.
But in practice, it turns out that it is very difficult to efficiently transfer energy between fast and slow factors. So, for example, if we put ideal gas particles in a rectangular container (or, alternatively, small hockey pucks on a rectangular air table), one of whose sides is connected to a spring (like a heavy piston), and we check the energy of the particles and the piston, we will find that over very long periods of time the energy will move cyclically between the particles and the piston (in other words, the temperature of the gas will change cyclically). That is, in this case, the energies do not reach, on average, an energy balance, and the lighter the particles - the longer the time when we see the cyclic behavior. It turns out that since the movement of the fast particles is ergodic (that is, the particles move "everywhere" so that, for example, the average energy of a particle's movement in time is equal to the average energy of the collection of all possible states of the particle), it is possible to superimpose their movement and obtain, for very long times, A conservation law ("adiabatic") which prevents the entire system from being ergodic. A similar problem, called the "infamous piston problem", was overlooked by the great physicists, and in 1999 it was presented as one of the open problems in statistical mechanics.
"A similar problem, called the 'infamous piston problem', was ignored by the great physicists, and in 1999 it was presented as one of the open problems in statistical mechanics"
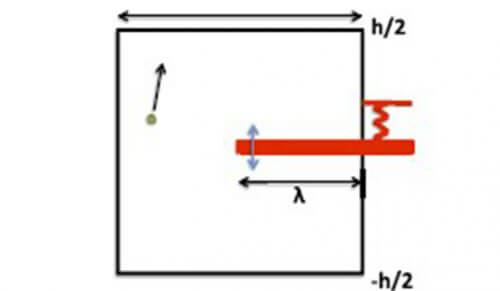
Prof. Rum-kidder rose, from the Department of Applied Mathematics, and its research partners, Prof. Kushal Shah (from IISE in Bhopal), Prof. Dmitry Toriev (from Imperial College), and Prof. Vassily Gelfreich (from the University of Warwick), sought to understand this phenomenon. They proposed to examine the movement of a light particle in different tables, which have a piston. This is how they were able to show that the transfer of energy between the particle and the piston is significantly accelerated if the particle is allowed to occasionally "choose", in a chaotic manner, which part of the box or container to be. That is, when the motion of the particle in the box is not ergodic for certain states of the piston. For example, if the piston is placed as a kind of shelf in a rectangular table, so that the particle can "choose" every time it reaches the shelf whether to move above it or below it, it turns out that the energy exchange becomes very efficient, and the particle and the shelf reach, on average, an energy equilibrium in a finite time, even when Their mass ratio tends to zero. In fact, the convergence to energy equilibrium is similar to that occurring in a random system that can be defined using only geometric data. Other boxes and tables, where the particle can spend part of the time in an area where it is not in contact with the piston, also behave in a similar way.

From all of this it is clear that the lack of ergodicity of the system of the fast particles can accelerate the ergodicity of the entire system. This surprising and non-intuitive conclusion is also true for many systems in which there is a separation of orders of magnitude (scales) between the fast movement and the slow movement.
this result Was published Recently in the scientific journal "Records of the American Academy of Sciences" (PNAS).
#Science_Numbers
In this study, the scientists calculated 1011 Collisions of particles or "hockey pucks". The calculation was performed over 4 days on a cluster of computers.
