New videos, filmed with an exposure of millions of billionths of a second, document protein drugs or photosynthesis processes in action - or while failing to act
- Proteins are in constant motion, and in the process they create chemical reactions that allow life to exist. This movement occurs on such a small scale and at such a high speed that even with a microscope it is impossible to follow it.
- Researchers use an x-ray laser that emits extremely short flashes, lasting millionths of a billionth of a second, to produce videos that show molecules in action and demonstrate how the structure of proteins changes during a chemical reaction between the molecules.
- These videos can record chemical reactions between biological molecules in unprecedented detail and show why drugs sometimes do not work on target proteins and how the process of photosynthesis in plants creates clean energy.
Deep under the foothills of the mountains, near Palo Alto, California, in an underground laboratory, the scientists rushed to finish the final preparations for a series of explosions. The experiment we planned, exploding tiny crystals of proteins, could reveal one of nature's best-kept secrets - the secret of the conversion of light into chemical energy in the process of photosynthesis in plants. The potential benefit: progress towards discovering an unlimited source of clean energy.
It was in December 2009, when a team of researchers and bleary-eyed students made days and nights at the US National Particle Accelerator Laboratory at Stanford University (SLAC) to prepare the experiment that was supposed to be conducted inX-ray laser (X-rays). In this facility, known as a "coherent light source in a linear accelerator" (LCLS), which is the most powerful facility of its kind in the world, accelerates electrons to speeds very close to the speed of light. One of the groups on the team was feverishly adjusting the devices that were meant to shoot the protein crystals into the x-ray beam. Another group claimed the crystal-firing devices of the so-called protein conjugate Light system I, one of the essential players in photosynthesis.
At the end of the particle accelerator tunnel, which is more than three kilometers long, the crystals began their journey towards the intense laser light. But even before each of them exploded, it was recorded with an innovative scientific technique, in a sort of flash photograph. This method holds promise for the future and opens a door for us to understand biological processes that occur on a very tiny scale, since it allows us to obtain a series of such flash images, with differences of femtoseconds, or millionths of a billionth of a second (10-15 seconds), and arrange them in sequence to make a whole video.
the physicist Richard Feynman He once said: "Everything that living things do can be understood in terms of atoms moving and oscillating." But never before could we directly witness the rapid movement of atoms and molecules in the living body. The method we developed, known as serial femtosecond crystallography (SFX), allows us to observe the super-fast "dance" movements of molecules, which determine, among other things, how drugs affect diseased cells and how chemical reactions convert chemical energy into other forms of energy.
Research teams around the world are already using SFX, and thanks to the method, scientists have been able to reveal the details of the process by which an experimental drug regulates blood pressure, thus paving the way for the development of more effective drugs to treathigh blood pressure. Using SFX, the structure of an enzyme that destroys red blood cells of patients was also discoveredsleeping sickness, a fatal disease caused by parasites. The method also made it possible for the first time to observe the initial stages of photosynthesis, where water molecules split into hydrogen and oxygen.
But in 2009, in that underground laboratory, when flashes (pulses) of x-rays began to destroy the crystals we had carefully prepared for the experiment, our professional future was at stake. In those days, many scientists claimed that the SFX method would never work, and all our requests for research funding were rejected. But then suddenly beautiful pictures appeared on the computer monitors in the laboratory X-ray scattering. We still remember, as if it were today, how we burst into rapturous exclamations of joy as we watched the birth of what was to be revealed as a new scientific field of the use of X-rays.
X-ray vision
Even before we developed the SFX method, scientists had incredible achievements when they were able to discover changes in certain chemical structures, but at that time they could not actually observe the most complex and delicate biological structures in action. For example, in the 80s, the late Nobel laureate chemist developed Ahmed H. Zawil, a method for tracking the movement of atoms during chemical reactions, using very fast flashes of a laser in visible light. But the wavelength of light seems to be too long to allow viewing the smallest structural details of proteins. And not long ago, dramatic advances in the field of microscopic technology yielded images of proteins and viruses with a resolution close to atomic resolution. But these images are not obtained at a high enough rate to capture chemical reactions that occur at lightning speed, such as those that occur in photosynthesis.
We therefore decided to use x-rays, which provide high enough speed and resolution to record chemical reactions between biological molecules as they occur. The key to our work was the development of technology that would make it possible to utilize the x-rays to obtain flash photographs of the molecules in a fraction of a second before those rays destroy the molecules. Scientists engaged in research in this field using the traditional method usually invest a lot of effort incrystal growth of proteins and other molecules in order to map the positions of the atoms in the molecules. Next, they expose the crystals to an x-ray beam and record the scattering pattern or ecircumvention of the rays when they hit the crystals. The molecules in crystals are arranged in a uniform internal order, in a repeating basic structure, so that the x-rays that hit them are scattered in predictable ways, which allows scientists to determine the positions and identities of the atoms based on the scattering model. This method is called X-ray crystallography. The method we developed, serial femtosecond crystallography, is based on the same principle, but allows viewing the atomic structure at a much higher speed.
But in the end, x-rays destroy the molecules we are interested in observing. The common assumption was that the x-ray laser, which focuses high-energy x-rays into a powerful beam, would only make matters worse. The bright light of the laser alone can punch a hole in steel. If so, it was reasonable to assume that a fragile biological molecule would have no chance of surviving under these conditions. We had to find a way to preempt and capture an image in the femtosecond time frame before the expected damage from the x-rays occurred. For comparison, the difference between one femtosecond and one complete second is equal to the difference between one second and 32 million years.
The key to the SFX method lies in that unimaginably short fraction of time between the moment when the laser flash hits the molecule and the moment when the laser energy tears the electrons from their atoms in the molecule. Without electrons, the positively charged residues repel each other and the molecules explode.
And this is how the method works: first, we cause a chemical reaction between the molecules in order to create a tiny crystal. After that, we shoot a very high intensity x-ray beam at the crystal in a very short flash, but long enough to allow some of the x-rays to scatter as they hit the crystal, just before the beam's energy breaks up the molecules. A detector captures the scattering image of the x-rays and based on its shape we can deduce the type of atoms in the protein being tested and what their location is. Capturing images of a stream of protein crystals as they pass at different angles through the x-ray beam allows us to reconstruct the XNUMXD structure of the protein. And finally, we are able to sequence a series of images obtained at different points in time during a chemical reaction and produce a sort of video documenting the molecules in action.
A cohesive view
The first step towards the production of such videos documenting molecules in action was taken in 2000 when the biophysicists Janosz Hajedo וRichard Neutsche, who were then working at Uppsala University in Sweden, calculated and found that the time span from the moment the x-rays hit the molecules to the moment they start to explode is about 10 femtoseconds. Scientists therefore need to capture an image in a shorter period of time than that. In 2006 they succeeded Henry Chapman, who is currently engaged in research at the German electron accelerator DESY, and his colleagues to do just that. They used a "disperse then decompose" approach to capture a low-resolution photo of A tiny image that was burned on top of a surface of silicon nitride (Yes3N4) and in it are seen two human figures and the sun shining on them.
But could this method also be suitable for the imaging of delicate biological molecules? When we proposed to try the method, the scientific community was mostly skeptical. The first ten applications we submitted for research grants were all rejected. The skeptics argued that the flashes emitted from an x-ray laser are not short enough, that the protein crystals are too small to return any detectable signals, or that we can never know the orientation of the crystal when the x-ray flash hits it - information that is essential for determining the crystal's structure.
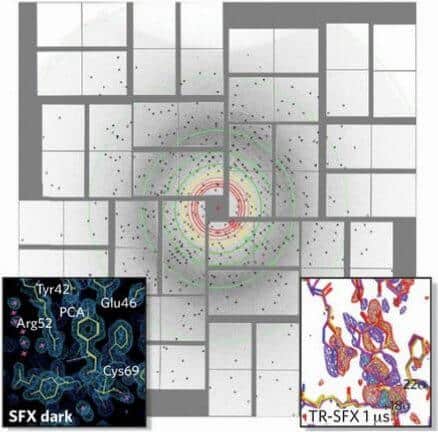
But we believed that if it is possible to photograph molecules of other types, as Chapman showed, it would be possible to photograph biological molecules as well. One of us (Petra Fromm) and her team members sought to prove the effectiveness of SFX in one of the most difficult experiments imaginable: with Or System I crystals. This protein complex, consisting of 36 proteins and more than 300 green and orange pigments light traps, is one of the most complex protein structures ever analyzed using x-rays.
Fromm knew the structure of light system I in its details, since for years before, she had been engaged in making crystals of this protein conjugate and determining its structure by other methods. And all of us, all the members of the research group, thought that the relatively large dimensions of the biomolecular coupling could actually be an advantage because even based on a small number of scattering models, we would be able to obtain an image, even if at a low resolution, in which it would be possible to identify the light system I. And this Indeed what we were able to do in that underground laboratory in 2009.
small is beautiful
To capture the desired flash image, we first had to prepare a pool of light system I crystals. Normally, crystallographic scientists grow large crystals to obtain sufficient x-ray scattering to reconstruct the protein structure. But in some proteins, the growth process of such crystals that excel in their uniform internal order may require many years of experiments. And as it has been proven, the process is almost not applicable for some of these proteins, including light system I.
Instead, in the SFX method, crystals of nanometric size (10-9 meter), which are much easier to grow in the laboratory. But the use of nano-crystals presented us with new challenges. We had to not only ensure receiving a sufficiently strong signal from such a tiny crystal, but also overcome some basic technical difficulties: how can we detect nano-crystals so tiny that they cannot be observed under a microscope? And how can we even place such crystals in front of x-ray flashes and even do it consistently 120 times a second?
Well, first we had to develop new ways of viewing our nanocrystals. One of the methods we implemented is called SONIC(Second-order nonlinear imaging of crystals Cyrillic), where the crystals convert two very fast flashes of infrared light into a single photon of green light. By the way, the nano crystals light up and glow like fireflies at night, which allows us to observe and identify them.
In another method we developed, we shoot the crystals into the x-ray beam one after the other, at a uniform and fast rate. One of our team members (Spence), along with two physicists from Arizona State University, Yves Weirstol וBruce Doak, built a device that works like an inkjet printer that shoots a jet of nano-crystal solution through the x-ray beam. This injection device fires the nanocrystals with such precision that they appear to march into the beam like soldiers in a line.
To prevent clogging of the injection device and blocking the flow of nano crystals, Wierstol was required to design a wide nozzle, yet also capable of passing a narrow jet of nano crystals. To do this, he injected helium gas around the outside of the nostril. The gas focused the jet of crystals to extremely tiny dimensions, down to a tiny particle the thickness of a human hair, while the diameter of the nozzle remained more than ten times larger.
After we prepared all the equipment required for the experiment, we faced another problem: how to deal with the mountains of data. Just one experiment can produce as many as 100 Terabyte of data, enough to fill the hard drives of 25 highly sophisticated desktop computers. And in order to create a XNUMXD image, we had to find the exact orientation of each of the crystals in tens of thousands of flash images and arrange them all in sequence so that we could see the image in its entirety. We have therefore developed a special software, in cooperation with Richard Kiryan וThomas White, who at the time were members of Chapman's team at DESY, which allows us to process the massive flow of data and produce accurate XNUMXD images of a molecule from it.
And so, step by step, we perfected our method. And thanks to her, in 2014 we were able to watch for the first time in real time the exchange of electrons between two key players in the photosynthesis process: the large protein couplet that captures sunlight, light system I, and a protein called Paradoxin.
When the light rays hit the light system I, their energy transfers electrons to the redoxin that carries them on to be used in the process of converting carbon dioxide (CO2) for biological molecules. As the ferredoxin completes its role in the electron transfer system and vacates the arena, the protein crystals are quickly dissociated, making it difficult to follow the chemical reaction. Only with the super-fast SFX method can you observe the changes that occur at lightning speed.
Our next challenge in this line of research, and one of the main topics that Fromm focuses on in her work as a biochemist, is the attempt to find out how the plant splits water molecules into hydrogen and oxygen with the help of sunlight and common metals. Splitting water molecules in the way it is done in plants may provide a source of cheap and clean hydrogen fuel for cars and power plants and enable the realization of the old dream of developing an economy based on renewable energy.
The videos we produce using SFX can pave the way not only for future breakthroughs, but also, in the near future, for the development of better drugs.
When we collected the first low-resolution images of the process of splitting the water molecules, we saw an initial hint of significant structural changes in the protein conjugate involved in the process, Light system II. And very recently, a research group led byJian-Ren Shen From Okayama University in Japan who applied the SFX method received even more detailed images of the process. Our goal now is to produce high-resolution images and organize them sequentially in videos that will show in detail all the steps in the process, at the atomic level, and thus reveal the secret of photosynthesis.
Targeted drugs
Now that scientists have started creating videos using SFX, these videos could pave the way not only for future breakthroughs, but also, in the near future, fordrug development better. We saw the potential inherent in them when we were engaged in researching the angiotensin receptor blockers (IIARBs . These drugs block the hormone cell receptor Angiotensin II, a hormone that causes blood vessels to constrict and increase blood pressure. The receptor blockers are used to treat high blood pressure, the main cause of stroke and heart failure in the US. And although the first generation of these drugs has been proven to be effective, the drugs bind to their target receptors only weakly and must be taken in high doses, which worsens their side effects, which include, among other things, headaches and dizziness and sometimes even more serious problems, such as swelling in the face and neck .
In the research we conducted, we discovered the reason for the loose connection between the drugs and the target receptors: it turns out that the drugs are not as well adapted to the receptor as they were supposed to be, so that many of their molecules do not fulfill their role. Based on more precise structures of the receptors we will be able to develop ARB drugs that will be more effective in regulating blood pressure. In fact, one such drug called ZD7155 is currently in the evaluation phase.
The more detailed and accurate information we have, the more effective drugs we can develop. The angiotensin II receptors belong to a very broad and important group of cellular receptors called G protein-coupled receptors. These molecules eembedded in the cell membrane allow the cell to sense and respond to its environment. The scientists who first deciphered the structure of the receptors in this group and their mechanism of action, Robert Lefkovich and Brian Kobilka, won theNobel Prize in Chemistry for 2012 On the groundbreaking discovery. Due to the essential role played by the G protein-coupled receptors in the growth and survival of cells, these receptors are considered key targets for new drugs. The ability to observe the changes that their structure undergoes will help chemists involved in drug development to design drugs that are adapted to the receptors and bind to them perfectly, thus preventing or reducing possible side effects.
"We showed that all the previous molecular models by which we hypothesized how a receptor and a drug bind were wrong in many important details," says Vadim Cherzov from the University of Southern California, who led the angiotensin II trial. For example, with the SFX method it was discovered that the structure of the receptors conjugated to G proteins at room temperature is different from their structure atCryogenic temperatures, very low, traditionally used in crystallography. This means that drugs designed to act on target receptors developed at extremely low temperatures below the freezing point will not work as required in the warm environment of the human body. (Sometimes, drugs act on more target molecules than those for which they are intended. This is, for example, the problem with drugs designed to treat sleeping sickness. The videos we produced show that the drugs act in a similar way both on proteins derived from the disease-causing parasite and on proteins in the cells of the human body. The more accurate images that we received help chemists develop drugs that affect the parasite's proteins only, and not proteins in the human body.)
The secret of sight
We are excited to see how other researchers apply the SFX method we developed to solve many other scientific mysteries. Marius Schmidt from the University of Wisconsin-Milwaukee and his colleagues recently used videos recording molecular activity to explain how the vision mechanism works. Normally, we don't attribute vision to bacteria, but it turns out that they are equipped withlight-receiving proteins which are the evolutionary precursors of those in our visual system. By capturing flash photographs at a higher speed than ever before, Schmidt's research team was able to produce a video that shows in slow motion events that occur at lightning speed, in fractions of a second, and reveals how the bacterial protein senses and responds to light.
The research team used SFX to capture images of the protein crystals as they pick up the light and react to it in time intervals of less than a trillionth of a second. The team was, specifically, mapping the atoms of the protein while in motion when a dye molecule contained in the protein turned yellow in response to light. This was the first time that scientists were able to capture the structure of the yellow dye molecule immediately after it absorbed the light and even before it reacted to it. This stage is essential for the absorption of light in all living things, including bacteria and plants, and is also the first stage in the vision process in humans.
Observing the way these light-receiving proteins react to light helps us understand how the visual system developed and even, more than that, allows us to follow closely, in unprecedented detail, the reaction between biological molecules, which takes place on the super-fast scale of chemistry. "This advances us by giant steps towards understanding the chemical processes underlying all forms of life," says Schmidt.
We believe that the future of protein crystallography, as well as our knowledge of nature, lies in the SFX method. And who knows, maybe within ten years, half of all protein structures known to us will no longer be presented just as static images on the pages of textbooks, but will be recorded in XNUMXD videos in action.

3 תגובות
Sounds amazing, and a lot of words, but where are the videos?
Why not bring one such video here as an example
For those who wondered why photosynthesis is "one of the secrets kept in nature" if every biology student studies the process - which means it is not a secret at all, I will explain:
The mystery is the specific system in photosynthesis that receives photons and transfers them to one central molecule (where photosynthesis continues) - the accuracy and efficiency of this specific system is so good that it is difficult to explain them with normal biological tools, and only through quantum theory can it be explained, or begin to be explained.