Weizmann Institute of Science scientists used new computational methods to identify a molecule with medicinal potential that would prevent the cell from receiving harmful messages - without disrupting the passage of vital messages
Our cells receive more messages than the average teenager's mobile device - and as with teenagers, it would be better to filter out some of them. The research group of Dr. Nir London from the Department of Organic Chemistry at the Weizmann Institute of Science, together with other colleagues, used new computational methods to identify a molecule that would prevent the cell from receiving harmful messages - without disrupting the reception of the messages that are vital to its function. The research findings were recently published in the scientific journal Cell - Chemical Biology.
When a signal is received through the outer surface of the cell and enters inside, the information is transmitted through proteins in one of the cell's signal transmission pathways until it reaches the nucleus. In most cases, these are messages that regulate the body's various functions, but these signals also play a role in diseases - starting with inflammation and autoimmune and metabolic diseases and ending with the growth factors that are disrupted in cancer. The pathway studied by Dr. London and his group, known by the initials JNK, mediates, among other things, messages that cause cell death following the accumulation of plaque in the brain in Alzheimer's disease and also participates in other inflammatory processes. The signaling molecules in this pathway - proteins from the kinase group - transmit their messages through "phosphorylation" (adding a phosphorylated group) to the next protein in the chain. The beginning of the pathway may include several kinase proteins, together called MAP3K, but the next link consists of only two proteins, MKK4 and MKK7, which transmit the message to the last link in the chain, the JNK protein - after which the entire pathway is named - and it transmits the message to transcription proteins which Genes in the nucleus are "turned on" and "turned off".
"This is the way many signal transduction pathways are built: a reduction to two protein kinases followed by a bottleneck of one protein," says Dr. London. "We used this feature to pinpoint the most suitable point for disrupting the process of transmitting the signal to the nucleus. The beginning of the pathway, which consists of five to ten MAP3K proteins, requires that they all be blocked. On the other hand, the JNK at the end of the pathway plays roles in all cells of the body, so disrupting its action will lead to significant side effects. Thus the MKK proteins are the 'golden link' in the chain. Drug treatment focused on one of them may reduce the impact of the harmful messages - without completely blocking the pathway."
Because the molecule binds covalently to the kinase, it inhibits the protein for its entire life. This means that if the molecule proves itself in clinical trials, it can be administered in small doses or infrequently."
The researchers focused on one of the proteins - MKK7 - a kinase lacking known inhibitors about which there is not enough research, among other things, because until now there was no way to separate its activity from that of its brother, MKK4. To their delight, they discovered in MKK7 the amino acid cysteine in an uncharacteristic position. Since cysteine can form strong and irreversible chemical bonds (in chemical terms, covalent bonds), a drug that would bind to this specific site could be very selective, meaning it would cause very few side effects.
In the past, Dr. London developed a computational method for discovering chemical compounds that covalently inhibit proteins. The method allows him to scan "in silico", that is, through computer simulation, huge libraries of molecules. The experimental equivalent of this method, based on robotic mechanization and libraries of real compounds in the laboratory, requires enormous time, infrastructure and resources, while on a computer it is possible to scan hundreds of thousands of molecules at a time. What's more: the method makes it possible to examine not only existing molecules, but also those that do not yet exist.
Research student Amit Shraga and other members of Dr. London's research group collaborated in the study with Dr. Ziv Shulman's research group from the Department of Immunology; with Dr. Bruce Lepker and Dr. Chakrapani Subramaniam, visiting scientists from the Pfizer Institute for Research and Development in the United States, and with their colleagues at the Nancy and Stephen Grand National Center for Personalized Medicine on the institute's campus; With Prof. Takeyoshi from Osaka University, Japan, and with Dr. Robert Hodkins from "Teva" company.
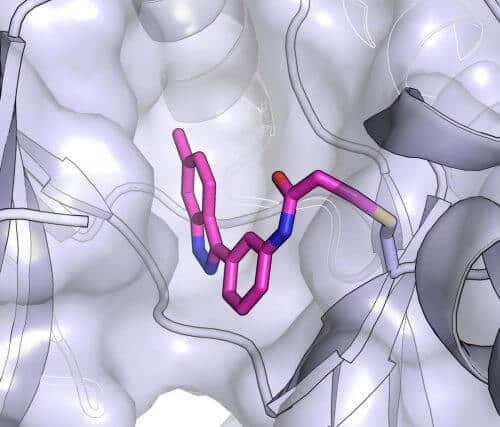
After identifying about ten promising substances through computer simulations, the researchers turned to testing them in the laboratory. Three of them worked well - but only one bound to the kinase with extraordinary efficiency. Using the software, the research team created a "binding model" to understand exactly how the inhibitor binds to the protein. According to the model, the scientists created different variations of the inhibitor to check if it is possible to add and improve. Finally, in collaboration with the Japanese laboratory, the researchers created a crystal of the molecule together with its target kinase - a sort of photograph of the interaction, but with atomic resolution. They found that the actual binding site was exactly as the model predicted.
The molecule identified by Dr. London and the members of his group may have medical applications in preventing the unwanted death of cells in Alzheimer's, in reducing inflammation in inflammatory bowel diseases and diabetes. "Since the molecule binds covalently to the kinase, it inhibits the protein throughout its life. This means that if the molecule proves itself in clinical trials - which are still very far away - it can be administered in small doses or infrequently," says Dr. London. The "Yade" company, the applications arm of the institute, submitted a patent application for the molecule.
