Devices that automatically regulate blood sugar levels are in the final stages of development before they are released to the market
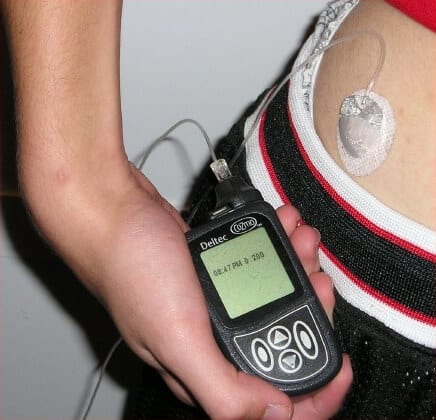
People with type 1 diabetes, whose pancreas does not produce the hormone insulin, must be vigilant and closely monitor their blood sugar (glucose) levels. A chronic state of high sugar levels, resulting from too low a level of insulin, may lead to damage to various organs and the nervous system, while too low levels of sugar may cause seizures or death. The best possible treatment now involves a device that continuously monitors blood glucose with a sensor inserted under the skin, an insulin pump (an external device worn on the body that can be programmed to release varying amounts of insulin), and a lot of trial and error on the user's part, since the sensor and pump are not communicate with each other.
Researchers are trying to make it easier for patients by combining and mechanizing the steps in the process. The end result, an artificial pancreas, is a system that can determine the amount of insulin required close to real time and release the required amount by itself. "The artificial pancreas will allow us to live an almost normal life until a cure for the disease is found," says Kelly Dunkling Riley, a registered nurse and diabetes instructor who participated in a clinical trial of an artificial pancreas called iLet, recently conducted by the company that develops it.Beta Bionics, in Boston. "For the first time in 24 years of being a diabetic, I could exercise whenever I wanted and work with my patients without the constant fear of hypoglycemia (a sharp drop in blood sugar)." After more than a decade of development, several artificial heart ventures are moving towards the final stages before they go on the market for widespread distribution:
In June 2016, a company submitted Medtronic, which manufactures medical devices, an official request to the US Food and Drug Administration (FDA) for approval"Closed hybrid loop": an insulin pump that analyzes data received from the sensor that continuously checks the blood glucose level and automatically adjusts the rate of insulin release. Users will still need to enter the insulin dose into the device to match meals. In May 2016, a study was completed that followed 124 diabetic patients who used Medtronic's device and showed that the system is safe and reliable in automatically determining the required doses.
At the beginning of 2016, one of the largest clinical trials to date was launched that examined 240 patients from all over the US and Europe. The experiment conducted by researchers from the University of Virginia, Harvard University and a consortium of organizations will test the level of safety and effectiveness of a system that combines an insulin pump, a continuous glucose sensor and a mobile phone. The phone runs software that relies on an algorithm that analyzes the blood sugar readings and tells the pump how much insulin to release. In the experiment, two different algorithms will be tested.
The company Beta Bionics is developing a device that monitors not only the administration of insulin, but also administration Glucagon, the hormone that raises the blood glucose level. Based on the data received from the continuous glucose sensor, the algorithm decides which of the hormones to release and in what dose. "The use of both hormones enables a tighter control of blood sugar levels," says the company's CEO Edward Damiano. He hopes to begin clinical trials in mid-2017. A version of the device, which works with insulin only, may receive approval as early as early 2018.
.

One response
And it is worth mentioning that the Trump administration is currently fighting the excessive bureaucracy of the FDA for faster approval of medicines and medical equipment for patients who need it.