By expanding the function of the genetic code, the German scientists were able to force bacterial cells to produce customized proteins on demand that include synthetic functional groups.
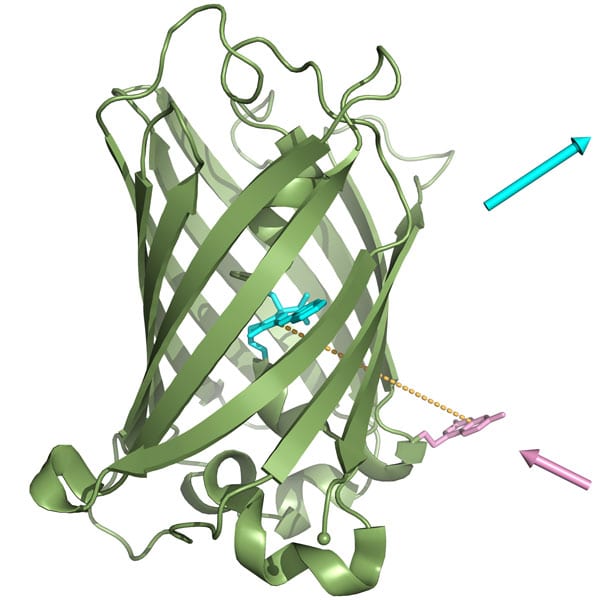
Proteins are the most important functional biomolecules in scientific research in the fields of life sciences, biotechnology and medicine. Following this, the question arises, how can these proteins be changed in the most efficient way in order to obtain certain required properties? In the past, these changes were usually made chemically or through genetic engineering. The research team of Professor Arne Skerra from the Department of Biological Chemistry at the Technical University of Munich (TUM) has now developed an extremely elegant integrated solution: by extending the function of the genetic code, the scientists were able to force bacterial cells to produce customized proteins on demand that include synthetic functional groups. To test the effectiveness of their idea, the scientists tried to pass a particularly challenging barrier: inserting an unnatural amino acid into a defined position within a natural protein, which is widely used today. In the field of biological research, this protein is known as "GFP" (green fluorescent protein The term on Wikipedia). This protein emits a bright green light and originates from the jellyfish that uses it to illuminate itself in the darkness of the sea depths. The team chose the dye coumarin (a chemical used as a flavoring agent and in the perfume industry) derived from the lavender flower as the synthetic group used as the side chain of an unnatural amino acid. The scientists "fed" this amino acid to a culture of the bacterium Escherichia coli - the microorganism "workhorse" of genetic engineering, and whose close strains are also found in the human intestines. Since the research team transferred the genetic program passed on to the bacterium - including its vital biosynthesis capabilities - the coumarin amino acid was integrated in a very specific position in the luminescent protein. This specific location in the protein was carefully chosen, explains the lead researcher: "We placed the synthetic amino acid at a distance very close to the center of light of the natural protein." They determined the distance between the modified chemical dye and the blue-green biological dye of the natural jellyfish protein in such a way that the interaction between the two dyes led to a completely new type of luminous fused molecule. Due to the multiple proximity between the two glowing chemical groups it was no longer possible to identify the light lavender color of the synthetic amino acid; Instead, it was the typical blue-green color of the luminescent protein that dominated. "The special thing in our case, and which is different from the natural protein (GFP), is that thanks to the synthetic amino acid that was inserted into it, the fluorescence can be initiated using a normal commercial light bulb instead of using special and expensive laser equipment," explains one of the researchers involved in the work. According to the lead researcher, the design principle of the innovative biomolecule, which is characterized by a particularly wide and challenging wavelength gap between the emitted light and the excitation light, should advance science towards the development of a number of fascinating applications. "We have now demonstrated that our technology works properly. Our strategy will enable the preparation of luminescent proteins on demand in a variety of colors for a multitude of future purposes."
