A production method developed at the Technion provides an "energy injection" for the production of hydrogen fuel using solar energy. The only by-product emitted into the environment is water
A study conducted at the Technion in the last year may give a significant boost to the use of clean and renewable hydrogen fuel. The research, conducted by Dr. Gideon Segev in the Faculty of Materials Science and Engineering, was published in Advanced Energy Materials, the third most important journal in the fields of energy and fuel. Dr. Segev, a post-doctoral student in Prof. Avner Rothschild's research group, completed his master's and master's degrees at Tel Aviv University, and is currently involved in developing methods for breaking down water using solar energy.
The development of renewable and sustainable energy sources has accelerated in recent years, due to sharp fluctuations in oil prices, growing awareness of pollution, and destructive environmental effects resulting from the use of hydrocarbon fuel. The hydrogen gas is a fuel for everything, which may replace hydrocarbon fuel without harming the environment because its combustion product is water.
Despite this, hydrogen production technology is still in its infancy due to limitations that have not yet been resolved. Unlike hydrocarbon fuel, which is easily produced from natural minerals of coal, oil and natural gas, hydrogen must be produced from other substances, such as water, that contain hydrogen as part of their chemical composition. The hydrogen production process is expensive, since breaking the chemical bonds in the starting material (water) consumes a lot of energy. Another difficulty is in storing the hydrogen gas.
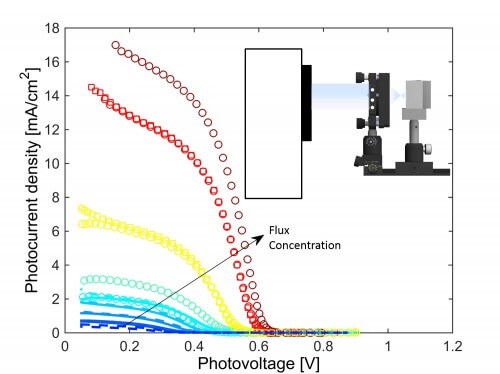
The research being published now describes a method that may significantly improve the process of producing hydrogen from renewable sources, that is, from water and solar energy. "The energy required to break down water to produce hydrogen comes from the sun," explains Dr. Segev, "and we are specifically researching the use of concentrated solar energy for this purpose. This means that instead of each solar cell absorbing only the tiny radiation that hits it, it receives a large dose of radiation that is focused on it through mirrors and lenses."
Today it is already known that the concentrated radiation increases not only the total energy reaching the cell but also the efficiency of the cell, that is - each photon of light is better utilized. The research now being published reveals that this phenomenon, which also exists in 'normal' solar cells, is much more dramatic when it comes to photoelectrochemical cells that break down water into hydrogen and oxygen. "This technology allows optimal utilization of the sun's radiation during the day to create hydrogen, and the use of hydrogen energy during the hours of darkness or at any other time. In other words, here is also a solution to the problem of storing solar energy, which changes by nature during the day and night."
The good rust
The sun sends to the earth, every hour, energy equal to the annual electricity consumption of the world's population. Therefore, it is a natural source of energy that will over time replace the mineral fuel (coal, oil and natural gas). Relying on mineral fuel exacts a huge price in pollution, disease and the destruction of nature and the environment, as well as a socio-economic price that originates from the fact that these minerals belong, for the most part, to a tiny number of people and private companies. On the other hand, the sources of renewable energy such as sun and wind are available to every entrepreneur, and the same is true for hydrogen produced by splitting water using solar energy.
For decades, various materials have been tested as candidates for the production of photoelectrodes for splitting water for the production of hydrogen. These materials are required to fulfill several necessary conditions: they must absorb solar radiation efficiently; They must break down the water effectively; And they themselves must not break down in water. In addition, it is highly desirable that they be available and cheap. In Dr. Chen Dotan's doctoral thesis, under the guidance of Prof. Rothschild, Dotan showed that trapping light in nanometric layers of iron oxide ("rust") results in the efficient decomposition of water and the creation of hydrogen and oxygen. The rust is stable in water and does not change its properties during their decomposition.
The current study strengthens the previous discovery and opens a new way to further improve solar cells based on iron oxide. The research, funded by the European Research Council (ERC), was mostly conducted in the hydrogen laboratory at the Technion, which was established with the help of a generous donation from the Adelis Foundation, as well as from the budget of the Israeli Center for Excellence in Solar Fuels Research.

One response
The energy received from the sun's radiation is subject to change such as: weather conditions, changing the angle of the sun's radiation and a certain month of the year, but in the same way the solar cells are also limited in the amount of their maximum power.
So the efficiency varies accordingly.
Beyond the electricity consumption spent on breaking down the water molecule, it is also necessary to invest energy to compress the hydrogen.
So the energy invested is greater than the energy received.