At the base of the research is the assumption that at least one of the causes of aging lies in the shortening of the telomeres.
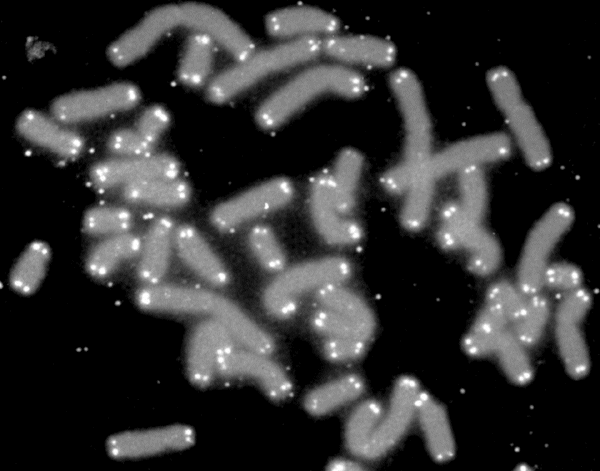
From time immemorial man has searched for the fountain of youth, one sip of which will erase the ravages of age. Now, we may have found an unusual type of such a spring, which can return mice to their happy youth... but only if they were damaged in the first place.
At the base of the research is the assumption that at least one of the causes of aging lies in the shortening of the telomeres. Telomeres are similar to the plastic ends of shoelaces. They are found in all the cells and are used as a stopwatch. Every time the cell divides, the telomeres within it shorten. When they become too short, the cell kills itself, or cannot continue dividing. Because of this, many in the scientific community believe that telomeres serve as a clock that measures human age, and as a natural barrier to eternal life. In a newborn baby, the long telomeres are just beginning to count. In a ninety-year-old man, the telomeres have already reached half their length and the clock is running out.
What will be the fate, then, of mice that are born with extremely short telomeres? Researchers from Harvard Medical School decided to put the question to the test. They used advanced methods of genetic engineering to create mouse pups with extremely short telomeres. The mice matured - or aged - at an extremely fast rate. Their organs degenerated, the nerve cells in their brains died, their DNA was filled with damage and the mice passed away at a relatively young age. This was the fate of the control group.
If this were the end of the research, there would be no great surprise. But the investigators had a card up their sleeve. Some of the mice were engineered with a 'switch' in the DNA. When the switch was turned on, it caused the production of an enzyme called telomerase, which is able to lengthen the telomeres and return them to a normal, 'young' state. The enzyme was supposed to, in fact, restore to the cells the youth they never had.
Four weeks after activating the enzyme in the mice, the experiment was crowned with success. The telomerase managed to re-lengthen the telomeres in the cells, and the results did not hesitate to come. The DNA damage decreased and many tissues in the mice's bodies stopped degenerating. The activation of the enzyme stopped the damage caused to the testicles and intestines, and in some cases caused the tissue to regenerate and restore its original activity. Brain cells woke up from the sleep imposed on them and began to divide and replenish the number of nerve cells lost during old age. The new cells immediately played a role in restoring neural activity in the brain, and at least some of them 'restarted' the olfactory processing system in the brain and allowed the elderly mice to experience and enjoy their surroundings again.
Apparently this is a real fountain of youth, but it is important to remember the caveats. First, there is no certainty that the telomeres are responsible for the aging phenomena. In fact, most people age and die before their telomeres reach their end. Second, the researchers did not cure old age, but the damage that was intentionally caused to the telomeres and affected the entire body.
But what if the telomeres are indeed responsible for aging? Will the day come when we can use the power of genetic engineering to insert the telomerase enzyme into all the cells in the body? In principle, this is possible, but biomedical technology is a decade or more away from this. Even if the enzyme is introduced into the body, it will need to be used very carefully. Because telomerase allows cells to live forever, it plays an important role in almost all cancers known to man. Irresponsible activation of the enzyme in the human body will inevitably lead to a jump in cancer incidence.
What will be our fate in that strange future that awaits us in a few decades? Can we gamble on the chance of eternal life, or risk cancer? And maybe a way will be found to control the action of telomerase to exactly the right degree? These are all questions that will only be answered in the distant future - and I hope that all readers will survive the passing of the years and discover the answer.

24 תגובות
Telomere lengthening Preventing aging I would love to have my telomeres lengthened I would like to return to the age of 20 and stay 20 forever Come on friends I would love for you to give me a telomeres pill so that I won't be at the age of a grandmother why do I need it it's unnecessary I don't want to be a grandmother it's disrespectful and unnecessary to the health care system why do they need burdens? There is a possibility that you will develop a vaccine for old age. I would be happy to be the first to try lengthening telomeres. Of course, check the stability before the experiment.
If anyone has more information about telomeres and the cause of aging, I would love to receive a "link" on the subject.
Hello pharynx
Could it be that you only have a link to the website of the worm experiment you mentioned? Do you perhaps remember how much his age was extended? And another question: What is the reason why it is not possible to qualify the same enzyme that was successful in worms as you said, in mice or in humans?
Thank you
Telomeres shorten due to cellular DNA replication. The telomeres are long sequences located at the ends of the two DNA helices. This is the natural process of the cells, when this leads to the "aging" of the cells. The telomerase lengthens the DNA to prevent the aging process.
I remember hearing about an experiment with worms that the extension of the DNA itself extended their lives significantly.
It makes perfect sense that inductively activated telomerase would result in an organism's life extension.
Hello Roy
In the abstract you wrote "Cells in the brain woke up from the sleep that was imposed on them and began to divide and replenish the number of nerve cells that were lost during old age" - how did you check the change in brain volume? And what did you mean by the word "sleep"? Were there brain areas where polyparation was seen more (you said the XNUMX-year-old mice went back to sniffing happily)? Have you checked which types of nerves are divided?
I would also like to receive the title of the article dealing with Ashkenazi Jews and long telomeres.
Thanks!
Shay –
The telomeres re-lengthen to the initial state in the embryo. There is at least one gene that plays a role in determining this initial length.
Zarathustra -
There is no computer simulation that can come close to animal testing. This experiment reveals another piece in the puzzle of aging. It may not be big, but it's there.
Shamil –
The reference is to the original scientific article, but those who are not Nature subscribers cannot read the whole thing but only the abstract. The record I wrote here is the fruit of my pen and was produced after reading the original scientific article.
The reference at the end of the article is not exactly to the article but only to the abstract, the full article is only open for a (high) fee to "Nature" subscribers. The point is that the article found here is nothing like an abstract. Is it the product ofRoey Tsezana's pen and we read from his thoughts after he read the summary, or did he bring us more than the article that was hidden from us and that he did read? I'll be happy to know.
15 and 13
There is a theory (sorry I don't have sources to bring you)
which states that the 'goal' of evolution is to 'preserve life' (possible even without the quotation marks).
That is, any way that preserves 'life' is legitimate in terms of evolution.
If the telomeres shorten during cell division - is it possible that they shorten from generation to generation (during the division of the reproductive cells) or are they regrown to the initial state in the embryo?
It is also interesting what exactly is the "initial state" and if we engineer mice with several times longer telomeres - will their lifespan be several times longer than that of normal mice.
For respondent 13, nature protects evolution so that the parents do not compete with the offspring for resources (food and living area)
A lot of noise and ringing about an imaging experiment.
They could just as well have done a computer simulation only that no one would have clapped.
None of the researchers have a real answer to what the aging process is and how it is related, if at all, to the aforementioned experiment.
When it comes to processes consisting of a huge collection of parts, science does not yet have the tools to decipher complex assemblies on such large scales.
There are no tools to decipher how consciousness grows in the brain just as there are no tools to decipher the connections between the four basic physical forces. Just as they don't know how to include all the prime numbers in one formula.
It starts to heat up and get closer to the thing itself, and the question that has already been asked is why didn't they directly try on naturally old mice or just those that are in the middle of life? Is the problem financial or did they try and the results were not successful and therefore were not published (is it possible in scientific research to shelve studies?)!
By the way, hasn't it happened to this day that a young cancer cell "mistakenly" sickens an old man before he started the disease itself?
I wonder why nature invented the telomere, what does it care if some live a long life! After all, eternal life is statistically almost impossible (when he will catch a fatal disease or be murdered, etc.) ..I wonder what is behind the existence of the telomere, what basic problems it solves, which we still don't see!
So is Ray Kurzweil keeping to his schedule? Is it possible to grind steaks? 20-30 years to last and we are in eternal life?
Crescent moon:
Everything you want appears in the link at the end of the article
Zarathustra,
The future is facing you
An uproar about something
Roy, could you please send the names of the researchers and where they published the research article and their name? I would very much like to look at it
Ofer,
As far as I know, old mice have not been given the enzyme. Or they gave and did not report.
Mike,
1. The telomeres are still there but they are shorter. Their harvest may be enough to explain some of the symptoms of old age. There was also an interesting study recently that shows that Ashkenazi Jews with longer telomeres live significantly longer.
2. I don't believe that overexpression of telomerase causes cancer, but it certainly enables cancer. The cells experience mutations all the time, and some will cause them to become cancerous. But in order for the cancer cell to survive and reproduce well, it needs to activate its telomerase. This is why more than 90% of cancers have a mutation that activates telomerase.
Mike:
What in the first question you assume is known is actually the opposite of what is really known:
http://en.wikipedia.org/wiki/Telomere
The image is taken from the original website:
http://antwrp.gsfc.nasa.gov/apod/
And clicking on the image leads to a link to the translated website.
And dear Roy Cezana: Thank you for the information. The details ("disappointing" a bit) of the experiment did not appear in the reports on the subject yesterday in the media.
Roy, thank you for bringing the study to our attention. I ask you 2 questions:
1) If it is known today that in very old people the telomeres still exist
What makes you think they play or may play some role in the aging of humans in general?
2) As far as I know, the accepted explanation is that cancer cells activate telomerase more to contribute to their ability to divide. On the other hand, I am not aware of a situation where overexpression of telomerase is a trigger for cancer. Do you know anything else?
The translation work is done voluntarily by the members of the Astronomical Club of Tel Aviv University, and they upload the translation by 11 am, so in the morning hours yesterday's picture may not have been replaced yet.
Why is there no link to the image of the day in astronomy on the page to the image that is found when you go to its link?
You expect something and get something else.
Why didn't they do the obvious and give the mice the enzyme when they were old? Or did they do it and not report the results?