The superheavy element isotopes with atomic numbers 104 to 114.
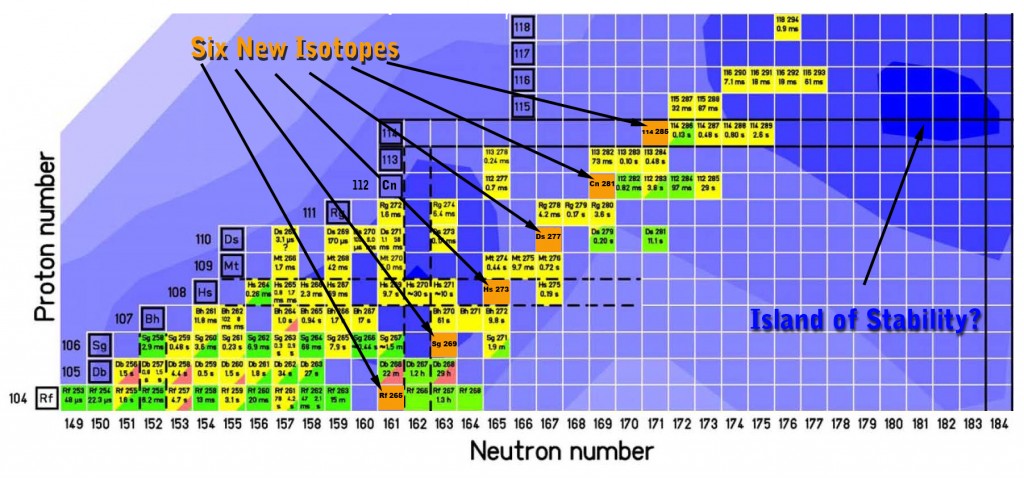
A team of scientists from the US Department of Energy's National Laboratory discovered six previously unseen isotopes of the super-heavy elements with atomic numbers 104 to 114. The beginning of the achievement came from the preparation of the new isotope number 114 (still unnamed), from which the researchers observed successive emissions of alpha particles which led to the acceptance of the new isotopes: copernicium (112), darmstadtium (110), hassium (108), seaborgium (106) and rutherfordium (104). Rutherfordium stops the decay chain when it eventually decays by spontaneous fission.
Information that will be gathered from the newly discovered isotopes will contribute to a better understanding of the theory of nuclear "layers", which is the basis of the predictions of the existence of an "island of stability", a collection of stable isotopes found between the "sea" of unstable, short-lived isotopes of the superelements. heavy
The team that discovered the six new isotopes was headed by Heino Nitsche, head of the Radiochemistry and Nucleus of Heavy Elements Group at Berkeley Laboratories and professor of chemistry at the University of California, Berkeley. The research findings were published in the scientific journal Physical Review Letters
"We tried to make super-heavy isotopes by injecting calcium-48 ions into a target of plutonium-242 atoms," notes the lead researcher. "It was a system quite similar to the one we used a year ago to verify the existence of element 114."
Nuclear stability is based on a layered structure - a model in which protons and neutrons are organized in increasing energy levels in the atomic nucleus. A nucleus with the outermost layer filled with protons or neutrons is described as a "magic" nucleus and is therefore stable. The possibility of finding "magic" or "doubly magic" isotopes of superheavy elements (in which the outermost layers of both protons and neutrons are completely filled) led to predictions of the existence of a field of increased stability in the XNUMXs.
The challenge is in the creation of isotopes by bombarding target nuclei rich in protons and neutrons with an ion beam having the appropriate number of protons to obtain elemental nuclei with desired properties.
The researchers point out that calcium 48 (48Ca), which contains a doubly magical layered structure (20 protons and 28 neutrons), "is particularly rich in neutrons and is capable of fusing with plutonium" - which contains 94 protons - "with a relatively low investment of energy to obtain a new nucleus." This is an excellent material for creating a nucleus of element 114."
Says one of the researchers: "There is a very low probability that two isotopes will react with each other to create a new nucleus. To do this, we need extremely powerful beams of calcium ions that will be intercepted towards the target, and then we need a suitable detector that can identify and find among the variety of reaction products our desired nucleus according to its unique decay pattern."
For many years, scientists believe that element 114 itself is located on an island of stability. Standard models predict that if an isotope of element 114 with 184 neutrons could be created, it would be doubly magical when both its proton and neutron shells are full, and it would be expected to have a long lifetime. However, current models predict that the magic number of protons should be 120 or 126. So the location of element 114 within the stable domain is currently in doubt. "Probably we won't be able to produce an isotope of element 114 until we build more powerful and efficient ion accelerators," notes the lead researcher. "But in the meantime, we are able to learn a lot about the nuclear layer model by comparing theoretical predictions to observations of the isotopes we can produce."
The research team that verified the existence of element 114 noticed the nuclei of two types of isotopes: 114(286) and 114(287), which decayed within a tenth of a second and half of a second, respectively. In scientific collaboration with a research group from Germany (GSI Helmholtz Center) two more isotopes were created: 114(288) and 114(289); These isotopes decayed for a time of two-thirds of a second and two seconds, respectively.
Although these lifetimes are not that long, they are long enough for the spontaneous fission that ends the alpha decay series. Alpha particles contain two protons and two neutrons - actually a nucleus of the element helium - and many heavy nuclei often decay through the emission of alpha particles to form atoms with a lower number of two protons. In contrast, spontaneous fission leads to much lighter fragments.
This year the research group at Berkeley decided to change their approach and prepare new isotopes using a unique method: instead of trying to add new neutrons to element 114, they will try to look for isotopes with a lower number of neutrons. The lower half-lives of these isotopes could allow new isotopes to be obtained through alpha decay before the spontaneous fission process disturbs the decay chain.
"This was a very calculated approach," explains the researcher, "because we hoped to find the isotopes that originate from successive alpha decays down the main chain of the elements, where the relationships between the isotopic number, the structure of the layers, and their stability are much more understood. Using this information and by measuring the energy of the alpha decay, we hope to learn something about how accurate the predictions of the layer structure model and the heaviest elements are."
In this way, after more than three weeks of operating the beam, the researchers observed one chain of decays from the neutron-poor element 114 nuclei. The two new isotopes created by this method, 114(285) and copernicium (281), by its alpha decay, had lifetimes of less than a fifth of a second before emission of alpha particles. The third new isotope, darmstadtium (277), had a short lifetime of eight milliseconds. The other isotopes: Hassium (273) existed for a third of a second, while the isotope Seaborgium (269) existed for 3 minutes and five seconds before it emitted alpha particles. Eventually, after two and a half minutes, rutherfordium (265) decayed through spontaneous fission. Overall, the lead researcher notes, the predictions were quite close to the practical results obtained. The discovery of these six new isotopes is a significant step forward in understanding the principles governing atomic nuclei.
The news about the study
