In research on the disease, it was found that mutations in a protein called Cu/Zn Superoxide Dismutase, or SOD1 for short, damage the proper folding of the protein and therefore also damage intracellular organelles such as the mitochondria, which are the energy factories of the cell. The defective protein accumulates in the cell during the disease, and surprisingly it turns out that it is found not only in patients suffering from mutations in the gene that produces the protein, but also in other patients.
A study in which a new component was discovered which may be a therapeutic tool in the muscular dystrophy disease ALS, was recently published in the prestigious journal Neuron. The research was done in collaboration between researchers from Ben-Gurion University of the Negev and the University of California, San Diego.
ALS (abbreviation of Amyotrophic Lateral Sclerosis) is the most severe disease from the group of degenerative diseases that damage the motor neurons, which control muscle activity. The disease is also known as "Lou Gehrig's disease" after the American baseball player who suffered from the disease in the 30s. It is a rare and debilitating disease that damages the motor neurons through which the brain controls the activation of most of the voluntary muscles in the body. In ALS patients, these nerve cells are destroyed, so they are unable to activate the muscles. Gradually the muscles themselves weaken from lack of activity until they are completely paralyzed. During the course of the disease, the muscles responsible for activating the limbs and the muscles of swallowing, speech and breathing are gradually damaged, in an irregular order.
About ten percent of ALS cases are hereditary, so in one family there is more than one patient who suffers from the disease. In some cases of familial ALS, the genes responsible for the disease have been identified, which may help in the future in finding drugs for these patients. In the other cases, there is no way to predict in advance the appearance of the disease in a particular person and it is not known what causes it to break out.
In research on the disease, it was found that mutations in a protein called Cu/Zn Superoxide Dismutase, or SOD1 for short, damage the proper folding of the protein and therefore also damage intracellular organelles such as the mitochondria, which are the energy factories of the cell. The defective protein accumulates in the cell during the disease, and surprisingly it turns out that it is found not only in patients suffering from mutations in the gene that produces the protein, but also in other patients.
One of the most interesting questions that the researchers are trying to decipher is the specificity of this defective protein, which accumulates only in certain tissues of the body, even though the mutant protein is found in all tissues and cells. The hypothesis of the researchers is that other proteins, called "chaferons", which help the correct folding of SOD1, either exist at very low levels or do not function properly in the tissues affected by the disease.
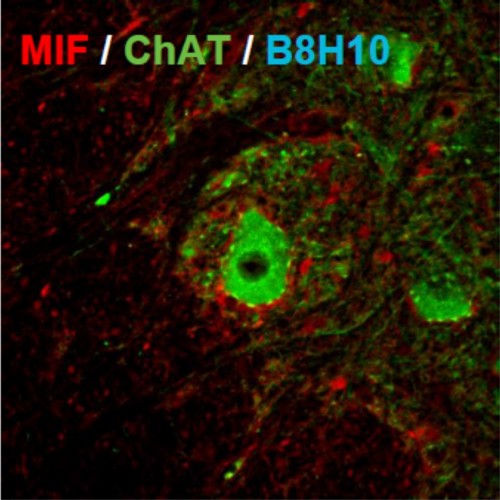
A new study, the result of a collaboration between the laboratory of Dr. Adrian Israelson from the Department of Physiology and Cell Biology at Ben-Gurion University of the Negev, the laboratory of Dr. Don Cleveland from the University of California San Diego and others, was recently published in the prestigious journal Neuron. The researchers identified a new activity that can inhibit the accumulation of the defective SOD1 protein. This protein was identified as macrophage migration inhibitory factor (MIF). The researchers showed that purified MIF protein prevents the accumulation of the defective SOD1 protein in the cell, and an increase in the expression level of MIF increases the vitality of various nerve cells, including motor nerve cells that express various mutants associated with ALS. The researchers also found that in the motor nerve cells, the protein level is very low, which may indicate an element of selective damage to these cells. An increase in MIF protein activity may be an attractive strategy for innovative treatment of ALS disease.
Dr. Israelson: "This research is a landmark, and has broad implications in understanding the mechanism of causing the degenerative disease ALS and its treatment. This enormous importance is embodied in the understanding of the fact that there is a growing involvement of the defective SOD1 as a common toxic mechanism in the ALS disease. This discovery has the potential to serve as a target for a therapeutic intervention aimed at increasing the level of MIF in the nervous system to treat ALS and other degenerative diseases that are associated with the accumulation of misfolded proteins."

3 תגובות
It's wonderful to see how science is perfected for him. Score: 90
Amazing only if it is possible to find a cure it would be great
Stunning