A team of chemists from Harvard University has developed an innovative microscopic method for observing, in color, non-fluorescent molecules at room temperature.
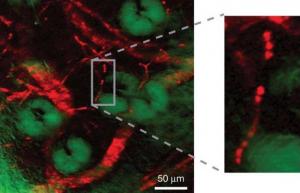
A team of chemists from Harvard University led by researcher X. Sunney Xie has developed an innovative microscopic method for the observation, in color, of non-fluorescent particles. The method, which is carried out at room temperature, allows researchers to identify patterns that could not be seen before in living organisms, and provides the ability to develop a variety of applications in biomedical imaging and scientific research.
Fluorescence is a phenomenon in which an electron in an atom absorbs additional energy from external light radiation and moves to a higher energy level, an excited level. Light energy exists in a unit called a photon. After a short stay in the excited state, the electron returns to its original energy level, the ground level, while emitting a photon. The energy of the emitted photon is in the wavelengths of light visible to the human eye, but this light exists for a very short time of one billionth of a second.
Many of the important colored biological particles, such as hemoglobin - a protein that transports oxygen and is found in red blood cells - absorb light but do not emit fluorescent radiation. In contrast, the electrons in these particles emit their excess energy by converting it to heat. "Since these particles do not emit radiation, they are practically invisible to contemporary optical microscopes," explains the lead researcher. In order to detect this type of separation in biological systems, the research team developed a state-of-the-art type of microscopy based on forced photon emission.
Forced emission of a photon was first described by Albert Einstein as early as 1917, and basically it is a process in which a quantum system passes from a high-energy state to a lower-energy state by emitting light as a result of interaction with the surrounding electric field. The emitted photon is exactly the same as the photons that caused the emission in terms of polarization, direction, frequency and incidence. The forced emission can be seen as a light amplification mechanism: one photon enters and two exit. This doubling is the basis of the operating principle of the laser, as well as of optical amplifiers.
The new microscopy method generates and records a forced emission signal through the use of two signal paths, input and output, which are coordinated with each other at very precise time intervals. In the input signal path, a modulator switches the intensity of the stimulating beam on/off at a frequency of five MHz. This modulation generates a forced emission signal at the same frequency. Each of these signals has a very short pulse duration of about two hundred femtoseconds. The signal emitted from these non-radiative particles originally provides a high-sensitivity image of particles that were previously invisible.
One of the several possible applications of this invention is the color mapping of the movement of non-radioactive drugs to their target cells. Another possible use is the imaging of extremely tiny structures such as blood vessels, including red blood cells and individual capillaries.
The structure and movement of the hemoglobin proteins in the various blood vessels play a significant role in many biomedical processes. Two examples of such processes are the transition of tumors from their dormant state to their malignant state and the supply of oxygen to the brain.
Existing medical imaging methods such as MRI and CT scanners either lack the spatial resolution necessary to view individual blood vessels or require external contrast agents. Fluorescent tags such as "green fluorescent protein" (GFP) are most common for observing the activity of biomolecules and identifying target molecules in cells. The method of labeling this protein provides very clear images. Despite this, the volumetric structure of the protein may interfere with the activity and delicate biological pathways, especially when it is larger than the tested bio-part. The research team was able to map the trajectory of non-radioactive drug particles and the detailed structure of blood vessels without the use of fluorescent tags. Their innovative method is also able to map the non-radioactive proteins in living cells of the bacterium Escherichia coli.
"While previous studies have used similar experiments to obtain spatially resolved images of irradiated furrows, our study, for the first time ever, used forced emission microscopy to image non-radiating furrows," explains one of the researchers on the team.
Although it is still necessary to overcome the possibility of photon damage, the complexity and the high cost of the system in order for it to be more applicable, "there is no doubt that our research provides a unique means of imaging a wide variety of animals, which are currently not suitable for viewing even with the most advanced optical microscopes," notes the researcher "This is just the beginning," adds the lead researcher. "Many interesting applications are expected to grow from this innovative tool."
The research findings were published in the scientific journal Nature.

One response
From the picture it seems that this is a resolution that revolves around a few microns and this is definitely an impressive increase compared to MRI which gives a resolution of about 40 microns. Still, these are very large structures, certainly not intracellular (with the exception of very large cells). A very interesting technology that I see many applications for in the context of environmental microbiology, a field in which I myself deal. I wonder how expensive such a device is (I'm guessing it should be around five million euros) and how quickly researchers will be convinced that there is a potential here that will return the investment. In the end it's a great invention but probably won't break too many boundaries in biology that needs sub-micron resolutions.