Using the method developed at the University of California at Berkeley using an atomic force microscope, chemists and chemistry students will be able to see with their own eyes the connection of atoms to molecules and the connections between them, and not just draw conclusions from spectroscopic analysis
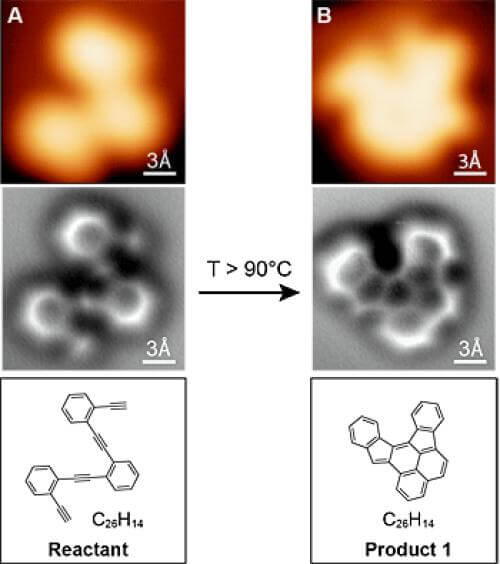
Every chemist's dream has come true - the ability to take atomic-scale pictures of a chemical before and after it reacts with other substances. All this thanks to a new technique developed by chemists and physicists at the University of California, Berkeley.
The scientists used a new generation atomic force microscope and photographed atom by atom, including an image of a chemical bond between atoms, and clearly saw how the structure of the molecule changes during a chemical reaction. Until now, the scientists could only draw the conclusions as to the nature of the process through spectroscopic analysis and not from direct observation.
"Even though I used these molecules on a daily basis, being able to actually see these images amazes me. Wow!” Said lead researcher Felix Fischer, professor of chemistry at Berkeley, "My teachers said we would never see it, and now it has come true."
Being able to photograph molecular reactions in this way will not only help chemistry students as they study structural chemistry and reactions, but it will also show chemists for the first time the product of their reaction and help them focus the reactions to get the product they want. Fisher, together with his partner Michael Chromy, professor of physics at Berkeley, took the pictures with the aim of building nanostructures of graphene, a field of research that is currently popular in the field of materials science due to its potential for application in the next generation of computers.
"However, the implication goes far beyond graphene." Said Fisher, "The technique can be applied, for example, in the study of heterogeneous catalysts." This technique is widely used in the oil industry and the chemical industry. Heterogeneous catalysts often include a metal such as platinum designed to accelerate chemical reactions, such as in the catalytic converter in cars.
"In order to understand the chemistry that takes place on the surface of the catalyst, we need a very selective tool that will reveal to us which bonds have been formed and which have been broken." He said, "The technique is unique at this stage and the precision it provides allows for structural information to be obtained. I think this is a breakthrough." Concluded.
"The atomic force microscope provides us with new information about the chemical bonds, which is very useful in understanding how different molecular structures compete and how they can be converted from one form to another." Says Karumi, "This may help us create new engineered nanostructures, such as interconnected networks of atoms that have a specific shape and structure for use in electronic devices. This advances us."

One response
Why for the first time? Can't you take a before and after picture of the same molecule?
Where can I get more pictures like this?