The ability to separate and purify certain particles from a chemical mixture is essential for the production of chemicals, however the existing distillation method is easy but requires a lot of energy
Engineers have developed an innovative method for making high-performance membranes from crystalline sieves called zeolites; The method could increase the energy efficiency of chemical separations up to fifty times more than current methods and offer higher production rates.
The ability to separate and purify certain particles from a chemical mixture is essential for the production of chemicals. Many industrial separation methods are based on distillation - a process that is easy to plan and implement, but consumes a lot of energy.
Researchers from the University of Minnesota, led by chemical engineer Michael Tsapatsis, reported the discovery in the last July issue of the scientific journal Science.
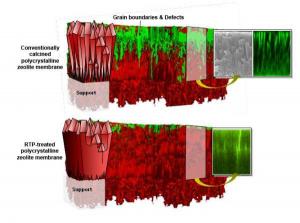
The research team developed a rapid heat treatment to remove structural defects in zeolite membranes - defects that limit the efficiency of their activity and delay the progress of this technology for decades.
"The use of membranes, instead of energy-intensive processes such as distillation and consolidation, may have a significant impact on the industry," said one of the researchers. This discovery could increase the energy efficiency of the production of important chemical solvents such as xylene, as well as renewable biofuels such as ethanol and butanol.
The researchers prepared zeolitic membranes by creating a layer of crystals into which organic ions were inserted that were intended to adjust the structure and the size of the pores of the crystal - two characteristics in zeolite responsible for the selection of materials that could pass through it. In the next step, they heated the zeolite layer in a calcination process to dissolve the ions and open the pores.
However, explains the lead researcher, "this method of creating zeolite layers sometimes leaves cracks in the boundary between the nuclei of the zeolite crystals." These defects prevented zeolite layers from being used effectively as membranes, because unwanted particles of various materials, which are rejected by the zeolite pores, are still able to penetrate through the defects in the membranes.
"Despite the fact that some of these defects can be repaired, the repair process is complex and expensive," explains the researcher. Today, zeolite membranes are used in unique, small-scale applications, such as the removal of water from coolants or other solvents.
In an attempt to reduce as much as possible the acceptance of these cracks and other defects, the rate of heating during combustion is extremely moderate, and the entire process may last up to forty hours - typically a material is heated at a rate of one degree Celsius per minute to a temperature between four hundred and five hundred degrees Celsius - and at this temperature the material remains for several hours until it is allowed to cool slowly. Since this heating process is energy-intensive and lengthy, until now it was complex and expensive to produce zeolite membranes on a large scale.
The research team developed an accelerated heating treatment called "Rapid Thermal Processing" (RTP), a process in which the zeolite layer is heated to seven hundred degrees Celsius in just one minute and remains at this temperature for no more than two minutes. Used as an annealing process, the new process "cleanses" the granular structure of the zeolite crystals from structural defects.
When the researchers examined the layers that went through the above process, they found no evidence of cracks in the boundary between the grains. Although they found other types of defects, they are sure that these do not affect the properties of the membrane or its performance.
Compared to zeolite membranes prepared by other methods, the researchers saw a significant improvement in the separation efficiency of the membranes that underwent the innovative treatment. Performing the process a second time, on the already treated material, improved the efficiency of the separation even more, to a level that is not at all known in the current industrial separation methods.
The researchers demonstrated their innovative method on relatively thin zeolite membranes - a few micrometers. They are now working on making zeolite membranes that are ten to a hundred times thinner in order to speed up the transit time of the appropriate molecules. They expect, eventually, to apply the new method and its advantages to these membranes as well.
