It is possible that the long-standing challenge of safe transportation of acetylene has been solved, in principle, thanks to a team of scientists from the National Institute of Standards and Technology in the USA
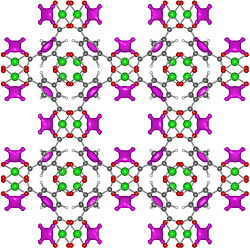
The long-standing challenge of safe transportation of acetylene may have been solved, in principle, thanks to a team of scientists from the US National Institute of Standards and Technology (NIST). The research team realized how a newly discovered material could be used to safely store the volatile material at low pressures in a hundred times greater quantity than other existing methods.
The team investigated the activity at the atomic level of a metal-organic framework (MOF) - a lattice-like structure composed of copper oxide and benzene - which is capable of adsorbing acetylene like a sponge. Using the institute's scientific tools, the scientists showed that exposed copper atoms, found within the lattice, are what give the material its ability to store acetylene. The new findings could help the chemical industry in the future, explains one of the scientists.
"This discovery could lead to significant savings in acetylene transportation costs," notes one of the researchers.
acetylene (The entry in Wikipedia), whose use has been widespread for decades in the fields of welding and lighting, is also important today as a starting material for the preparation of a variety of chemicals for the production of plastics and explosives. In the USA alone, hundreds of thousands of tons of acetylene are produced every year, but its high volatility makes its transportation problematic: it becomes dangerous for an explosion at a pressure of thirty psi, twice the normal atmospheric pressure in air. For the safe storage of acetylene, special storage cylinders are needed which additionally contain both a porous material and liquid solvents such as acetone.
The research team used a measuring device known as "powder neutron interference" and computerized results to examine one such material called HKUST-1, which contained a sponge-like internal structure in which copper atoms are exposed to air. The test showed that the acetylene particles bind to these exposed atoms thanks to weak electrical charges that allow the material to store about two hundred cc of acetylene in one gram of material at normal pressures - an amount close to the amount of similar chemicals, which are compressed in the storage cylinders at much higher pressures.
The lead researcher adds that this discovery could also help scientists gain a deeper understanding of MOF-type materials, which could be used to store other materials. "So far, more than a thousand new materials from this family have been produced," notes the researcher. "We hope that our method will turn out to be a good and quick way to examine the properties of such materials."

One response
A little more research and maybe this could be a method
Safer to drive LPG vehicles (and maybe also
one way or another in hydrogen).