Converting one chlorine atom to a hydrogen atom creates chloroform from this dangerous gas with a utilization close to XNUMX percent
Due to its toxicity and the danger involved in its maintenance, it is no longer possible to produce and use carbon tetrachloride in many countries. However, in the process used to prepare other chlorohydrocarbons, such as chloroform, carbon tetrachloride is also obtained as a by-product.
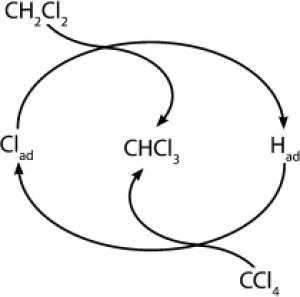
A research team led by the researcher Bert M. Weckhuysen from Utrecht University in the Netherlands, has now found a new and interesting approach that could lead to an efficient recycling of this material. As the researchers published in the journal Angewandte Chemie, a lanthanum chloride catalyst causes carbon tetrachloride and its "partner" in the reaction dichloromethane to convert one chlorine atom into a hydrogen atom and thereby create chloroform with a utilization close to XNUMX percent.
In order to increase the surface area of the catalyst, lanthanum chloride (LaCl3) was added to the substrate consisting of carbon nanofibers. This leads to the creation of a highly active, selective and stable catalyst that assists in the exchange of hydrogen atoms with chlorine atoms between carbon tetrachloride and dichloromethane. "Computer calculations show that the mechanism occurs through two separate exchange reactions," says the lead researcher. It appears that the surface of the catalyst contains not only the terminal chlorine atoms of the crystal lattice, but also other forms weakly associated with it. Dichloromethane replaces one of its hydrogen atoms with one such chlorine atom that is weakly bound to the surface. It leaves behind the hydrogen atom that binds weakly to the surface. This hydrogen atom can react with carbon tetrachloride, which in the reaction separates from one chlorine atom in favor of the catalyst.
Both steps of this reaction create chloroform exclusively - no by-products are accepted. This catalytic reaction is surprising in that until now it was assumed that the presence of oxygen - in a gas state or as atoms associated with the crystal lattice - is necessary for this type of reaction. Says the researcher: "We report here for the first time that a catalytic material based on lanthanum is able to activate both a carbon-hydrogen bond and a carbon-chlorine bond in the absence of oxygen."
