A new method makes it possible to use mass spectrometry for quality control of recombinant protein production - without the need for "purification" that requires a large investment of time and money

Spiritual teachers sometimes advise their students to turn disadvantages into advantages. Prof. Michal Sharon's group from the Department of Biomolecular Sciences at the Weizmann Institute of Science took this approach in the study of proteins. As reported in the scientific journal Communications Biology, the scientists have developed a method that significantly speeds up the quality control processes of producing proteins for the pharmaceutical industry and many other uses.
Prof. Sharon's laboratory deals with the analysis of protein structures using advanced methods of mass spectrometry - a technology that makes it possible to determine the composition of proteins down to the level of individual atoms. Until now, it was common to think that this technology has a significant limitation: when using it to test proteins originating from a whole cell, the proteins produced in large quantities overshadow everything else and make them "invisible". This fact is of course a disadvantage when you want to get a complete picture of all the proteins in the cell, but suppose we were interested in only one protein, which is produced in the cell in large quantities anyway? Let's take for example recombinant proteins, which are used in the food and pharmaceutical industry and are produced through "over-expression"; In this process, a gene that codes for the desired protein is injected into the cells, thus causing the cells to produce it in large quantities. Today, in order to test the quality of a protein produced in this way, the cell in which it was created must be killed, and the protein isolated in a process known as "purification".
This is exactly the stage where Prof. Sharon and her research colleagues turned the disadvantage into an advantage. If a protein that is produced in large quantities overshadows all the other proteins in the cell and makes them "invisible", this is a great opportunity to examine its properties using mass spectrometry of all cell proteins, thus saving the purification process that requires a large investment of time and money.
To test this, Dr. Gili Ben-Nisan, Shay Wimer and other researchers in Prof. Sharon's group inserted various genes into cells of yeast and insects, and even into human cells growing in culture, with the aim of making them produce a certain protein in larger quantities than usual. Later, we examined the protein using mass spectrometry, after purification and without purification, and compared the results.
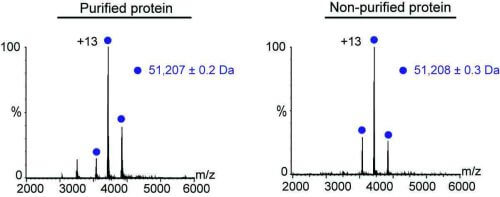
The test performed well, whether or not the protein was purified before its properties were tested. In both cases, the scientists were able to measure many properties of the protein, including the molecular weight, the way it folds, the solubility, the general structure and even the ability to bind to other biological molecules. However, while a test that included purification took between two and three days, the test conducted without purification lasted only about 30 minutes.
These findings indicate that mass spectrometry without prepurification can greatly simplify and accelerate the quality control process of proteins. This conclusion is particularly important considering the increasing scale of production of dedicated proteins. Since the creation, in 1982, of the first recombinant protein in history - insulin - dozens of other proteins have been created. In fact, since 2011, in the United States alone, more than 60 recombinant proteins have been approved for use as drugs for cancer and genetic, autoimmune and infectious diseases, including belimubab - an antibody for the treatment of lupus; glucarpidase - an enzyme given to cancer patients with impaired kidney function; and tbo-filgrastim - a growth factor that encourages the production of white blood cells. In addition, the vast majority of the industrial enzymes used in the paper industry, leather, detergents, fabrics, food and more - are recombinant proteins; In the food industry, recombinant proteins are used to process coffee, to produce vegetable and fruit juices, and as artificial sweeteners.
Dr. Shira Warshavsky and Dr. Sheral Fleishman from the Department of Biomolecular Sciences also participated in the study; Aliza Katz, Dr. Hadas Cohen-Dabashi and Dr. Ron Diskin from the Department of Structural Biology; Mittal Yona, Dr. Tamar Ongar and Dr. Yoav Peleg from the Department of Life Sciences Research Infrastructures; and Dr. David Morgenstern from the Israeli National Center for Personalized Medicine named after Nancy and Steven Grand.
A bacterial culture in a volume of 10 milliliters can produce up to 10 milligrams of recombinant protein during 24 hours.

2 תגובות
Is it suitable as a medicine for lymphoma patients
Is the protein suitable as a drug for lymphoma patients?