A team of chemical engineers recently identified the two main factors that determine the nature of the optimal catalyst for the conversion of atmospheric carbon dioxide into liquid fuel. The research itself will be able to help in the development of innovative catalysts that will be particularly effective and also cheap
[Translation by Dr. Nachmani Moshe]
A team of chemical engineers from the University of Pittsburgh recently identified the two main factors that determine the nature of the optimal catalyst used to convert atmospheric carbon dioxide into liquid fuel. The results of the research, published a long time ago in the scientific journal ACS Catalysis, will be able to help in the development of innovative catalysts that will be particularly effective and cheap.
Imagine an electricity generating plant that utilizes the excess amount of carbon dioxide that would have been emitted into the environment from the combustion of fossil fuels and converts it back into fuel. Now, imagine that the same plant uses only a small amount of water and energy from the sun's rays for its full operation. The plant will not burn fossil fuels and in fact will even reduce the amount of carbon dioxide emitted into the atmosphere during the production processes. For thousands of years such natural plants have used water, sunlight and carbon dioxide to create sugars which are raw materials for fuel. Scientists around the world are now adopting this mechanism of plants in nature to produce energy.
"We're trying to speed up the natural carbon cycle and make it more efficient," said Karl Johnson, a professor in the Department of Chemical Engineering at the University of Pittsburgh and the principal investigator. "There is no need to waste energy on all the addition required to grow plants, and the result will be a man-made carbon cycle that produces liquid fuel." However, there is one caveat - carbon dioxide is a very stable molecule, and therefore a considerable amount of energy is required to make it react. One way to use an excess amount of carbon dioxide, CO2, is to remove one oxygen atom and fuse the remaining molecule, carbon monoxide (CO) with a molecule of hydrogen (H2) to form methanol (CH3-OH). However, during this stage it is necessary to heat the backing to a temperature of 1000 degrees Celsius, a temperature that is difficult to maintain over time, especially when the only available source of energy is the sun.
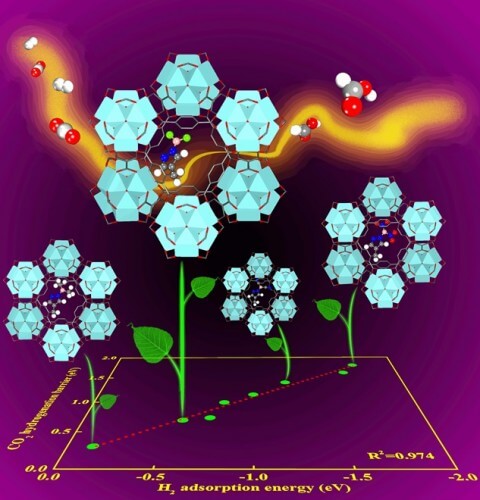
A catalyst may cause the carbon dioxide to participate in the reaction at much lower temperatures. Researchers are experimenting with different substances that cause carbon dioxide to split even at room temperature. However, these active catalysts, for the most part, are too expensive for use on an industrial scale, especially in light of comparing their prices with those of fossil fuels as a cheap energy source. The low price and widespread distribution of fossil fuels prevent many companies from investing in the expensive and challenging research of developing new catalysts. The study, titled: "Screening Lewis Pair Moieties for Catalytic Hydrogenation of CO2 in Functionalized UiO-66", provides researchers with a pretty good starting point as to the efficient search path for the optimal catalyst.
The researchers tested a series of eight different functional groups of Lewis acid-base pairs (Lewis pairs for short), which are very active compounds used as catalysts. They found two factors responsible for the effectiveness of the catalyst: (1) its hydrogen adsorption energy and (2) the hardness (a measure of the gap between the ionization potential and the electron affinity) of the Lewis pairs. In light of this conclusion, the researchers intend to work in collaboration with other researchers to find more efficient catalysts, which will hopefully bring the researchers closer to building plants to produce electricity in the form of liquid fuel while reducing the amount of carbon dioxide emissions.
The news about the study Article Summary
