Sharon Laffler is in the final stages of his doctoral thesis in the Department of Cell Research and Immunology in the Faculty of Life Sciences at Tel Aviv University. He carries out his research in the laboratory of Prof. Miguel Weil
I met with Sharon Lefler to ask him what is being done there at the university.
Sharon is in the final stages of his doctoral thesis in the Department of Cell Research and Immunology in the Faculty of Life Sciences at Tel Aviv University. He carries out his research in the laboratory of Prof. Miguel Weil.
Sharon, so what are you doing there?
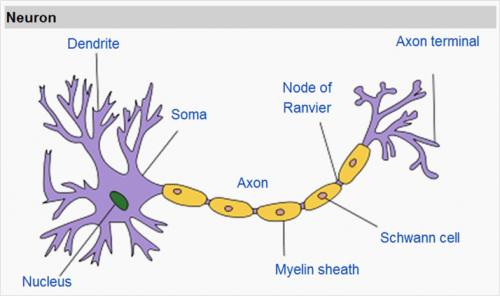
In general, the laboratory deals with the issue of embryonic development, i.e. the question of how a single cell (fertilized egg) develops into a complete embryo containing many and varied types of cells. Another subject we deal with is neural differentiation, that is, the differentiation of embryonic stem cells into nerve cells, and the study of diseases that cause damage to the nervous system.
How do you study diseases of this type?
First, a research model is needed. Today, most laboratories deal with a murine model. The intention is that a disease is induced in a mouse with the help of mutation or drug treatment, when it must be remembered that these diseases are currently known only in humans. The problem with this model is that the mouse is not exactly human, and its genes 'behave' differently. On the other hand, the disease cannot be studied directly in humans because it is not possible to test the nerve cells in the patient's body or grow them in cell culture. The solution is the use of stem cells.
What are stem cells?
Our body is made up of very different tissue and cell types. A muscle cell is different from a nerve cell or a skin cell. This is despite the fact that the origin of all cells is one primary cell created by the union of the egg cell from the mother and a sperm cell from the father. The development process of the embryo includes division of the cells and differentiation into functions. A stem cell is a cell that has not yet differentiated and has the potential to become any type of cell.
How are the stem cells related to the development of an improved research model for diseases?
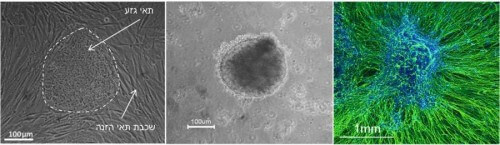
The first step is to obtain normal embryonic stem cells. The second step is to learn how to make the stem cells differentiate only into the type of cells we are interested in studying, nerve cells (see picture 2). At the same time, the third step is to similarly produce an in vitro embryonic stem cell line that contains the mutation.
So where do these cells come from?
First, it is worth mentioning that the research in embryonic stem cells is very problematic in many countries of the world because of the approach of the Christian religion which holds that from the moment of conception, there is a living being that must be protected. Judaism, on the other hand, does not hold this view. As far as Judaism is concerned, the fetus is considered a being only from the age of 40 days. This is one of the reasons that there is no principled prohibition to engage in stem cell research in Israel, subject, of course, to the Helsinki Committee.
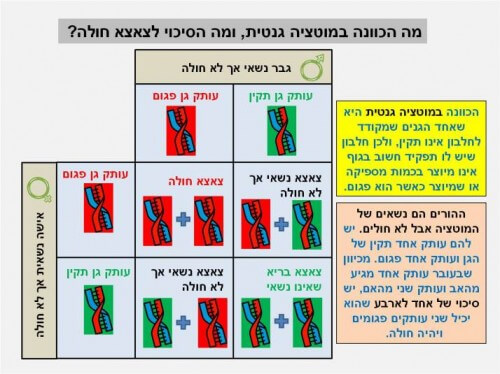
During artificial insemination, when the embryo contains only eight cells, one of them can be taken for genetic testing, so that the remaining seven cells can continue to develop in a healthy way.
When both parents are carriers of a genetic mutation related to a serious disease (for example, cystic fibrosis), the parents must perform artificial insemination, to make sure that the fetus is healthy. It is possible to check whether the same cell we took contains two copies of the defective gene (see Figure 3). If both copies are normal, the remaining seven cells can continue to develop in a healthy manner. If both copies carry a mutation, the remaining cells will not be used for pregnancy purposes, but they can be used for research purposes of the disease. The stem cells can be grown in a dish, when special treatments must be performed to keep them from differentiating.
What disease are you researching in the lab?
The disease is called familial dysautonomia (FD - Familial Dysautonomia) and it appears mainly in Jews in Israel and the USA who are of Ashkenazi descent from a certain region, and therefore it is very rare. The patients are born with problems both in the involuntary nervous system which is responsible in the body for, among other things, blood pressure regulation, body temperature regulation and digestion, and also in the sensory nervous system which is responsible for absorbing stimuli from the environment. In the past the disease was unknown and the sick babies did not survive, but today the knowledge base is greater and with the help of various treatments the sick live longer. Today we know how to identify the problematic gene, but we do not know what the functions of the defective protein that is found in every cell in the body are.
The stem cell line with the defective gene was produced by Prof. Binyamin Raubinoff from the Hadassah Ein Kerem Hospital. In collaboration with his laboratory, we developed a process, which takes about two months, and at the end of which the cells differentiate into the nerve cells that interest us. The use of these cells for the development of a research model is new, unique and groundbreaking on a global scale.
Later we had to repeat the process for the diseased cells, when we were not sure if the diseased cells could even develop into nerve cells. The results we received show that the cells in culture look like cells that develop from a healthy embryo, meaning that it is not a complete system failure in the development of nerve cells. Now we are still investigating the differences between the diseased and healthy cells in a number of different directions, but at the same time we have a model system ready for the next step.
What is the next step?
These days, a new robot is being installed in our laboratory that performs a drug screening process with the help of an automatic microscope. We feed the robot a large number of substances and it checks the reaction of cells to them. The robot is able to test a very large amount of materials and monitor a large number of parameters at a speed that is impossible for a human researcher. With the help of the model system we developed, we can find a problem in the diseased cells and then look for a substance that fixes it.
We know, for example, that people with familial dysautonomia have a low level of the FD protein, and there is even a substance of plant origin that helps, but the problem is that it is toxic. With the help of the robot, a large amount of derivatives of the substance can be tested on the model cells, hoping to find an effective and safe one that will increase the protein level in the cells. Such a drug could prevent the deterioration of the patients' condition, raise their standard of living and perhaps even more.
Isn't that what drug companies do?
Yes, but it must be remembered that this is a classic example of the concept of 'orphan diseases'. A company whose goal is economic profit does not have the incentive to invest so much money and time in finding a cure for such a rare disease with such a low number of patients. It should also be remembered that beyond finding a cure for the disease, while trying to understand the differences between a healthy neuron and a diseased one, we also gain a lot of basic biological knowledge, which can also contribute to the understanding of other neurological diseases.
----------------------
During this interview I chose to discuss only the scientific aspect of the subject, but there is also a personal aspect. Those who are interested, are invited to watch the Real Faces program on Channel 10 in which Prof. Miguel Weil told journalist Amnon Levy his personal story.
----------------------
The research was done in collaboration with and partially funded by the Israeli Association for Family Dysautonomy.
* The article on the subject, from which image 2 is taken:
* Valensi-Kurtz M1, Lefler S1, Cohen M, Aharonowiz M, Cohen-Kupiec R, Sheinin A, Ashery U, Reubinoff B, Weil M: "Enriched population of pns neurons derived from human embryonic stem cells as a platform for studying peripheral neuropathies”. PLoS One 2010;5:e9290.
-----------------
I would be happy to meet and talk with any research student (maybe you?) who is willing to participate and tell me a little about what he is doing (and all for the price of a not too long conversation). You can contact me via Contact Us Form.
It's time to tell everyone what you're doing, maybe this time they'll understand too
The article was published on Oren Shaya's blog.to a constant extent"

One response
I was very moved by Amnon Levy's article with a real face, God bless you.