A state-of-the-art material characterized in the National Laboratory of the Ministry of Energy in Spain could open a path towards more efficient fuel cells
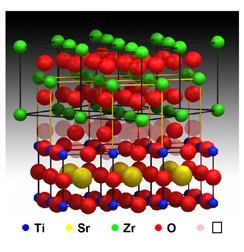
A supercrystal developed by scientists in Spain has improved the known ionic conductivity at a temperature close to room temperature by a factor of 100 million, while exhibiting "properties of enormous amplification in ionic conductivity," says researcher Maria Varela, who characterized the structure of the new material together with the researcher Stephen Pennycook.
The characterization was done using a Z-contrast transmission-scanning electron microscope with a power of 300 kV, which is able to achieve a corrected resolution-defocusing of light rays close to 0.6 Angstroms, until recently - a world record. The direct images show the crystal structure that explains the material's conductivity.
"It's amazing," says the researcher. "We are able to see the structure of the organized and compact interface that contains many pathways for ion conduction."
Fuel cell technology consisting of solid oxide requires ion-conducting materials - solid electrolytes - that allow oxygen ions to migrate from the cathode to the anode. However, existing materials lack atomic-scale cavities wide enough to provide a fast path for an ion, much larger than, for example, an electron.
"The new layered material solves the current problem by combining two materials with completely different crystal structures. The mismatch between these different structures leads to a distortion in the atomic arrangement of the interface layer between them and thus creates sufficiently wide channels that allow the ions to move quickly," explains the researcher.
In other materials used for fuel cells, on the other hand, the ions are "forced" to move in channels that are much narrower and have smaller spaces, so that their rate of movement slows down. Instead of forcing the ions to "jump" from hole to hole, the new material contains many large holes and thus the ions are much freer to move between the electrodes, which of course affects the overall conductivity.
Unlike previous materials used for fuel cells, which required a high temperature for the conduction of the ions, the new material maintains ionic conductivity even at a temperature close to room temperature. The need for high temperature was one of the barriers to the development of more efficient fuel cells.

12 תגובות
Marie:
An atom is electrically neutral and an ion carries a charge (positive or negative) and can also contain more parts than one atom.
For details, see here:
http://en.wikipedia.org/wiki/Ion
What is the difference between an ion and an atom?
lion,
The movement is of ions, not of electrons. It seems to me that you are confusing the processes that take place in the MLM, with the movement of holes.
In fact, the electrolyte must be non-conductive because then a short will happen
Shabbat Shalom.
lion:
As far as I know, it is actually the movement of whole pigeons.
Beyond the fact that the material used as an electrolyte is a different material than the one from which the ions were derived and your model cannot explain how in such an environment the first ion passes, it is written in the article that the whole point is the existence of gaps that are large enough for the ions to pass.
To Roy and my people
It seems to me, and Roy, tell me if you think it's true, that the movement of positive ions in the electrolyte is not actually a movement of the ions, but a movement of electrons in the opposite direction, that is, if there is a positive ion, an electron jumps to it from the adjacent atom, thereby becoming an ion. It's like in semiconductors talking about the movement of holes - which is a lack of electrons.
Note that the conductivity was improved by 8 orders of magnitude, not the ion velocity.
Speed depends on many other factors.
And in general, the efficiency of a fuel cell does not depend only on the speed of the ions passing through the medium.
The biggest problem today with this technology is storage.
The solutions offered today rely on high pressures (over 100 atmospheres)
And still manage to store enough fuel for only 100 km.
This changes the storage and transport of the dalclicker radically.
Let's start with the fact that my knowledge comes from batteries, but the principles are the same...
Regarding the first question, they use a solid and not an aqueous electrolyte, therefore these diffusion data are incorrect (on second reading, they did not say the medium for which they took the data), in addition, diffusion is an undirected statistical movement, here you have an electric field created due to the potential difference, which can increase speed (actually create directed movement).
By and large, the main problem is the order of magnitude of the diffusion coefficient, which is relatively low (compared to electronic conduction in an electrical wire). The diffusion coefficient depends on many things, for example as you said temp. You can also add the diameter of the ion that passes through the medium, attraction/repulsion forces between the ion and the electrolyte, the concentration of the ion in the electrolyte, the charge of the electrolyte if any, the structure of the electrolyte and many other things.
If you picture a solid electrolyte, it's like those games where you throw a coin into a machine full of pins and the coin falls between the pins and collides with them. The force of gravity that pushes the coin is parallel to the electric field and the distance between the pins simulates the size of the channels that the oxygen ion has to pass through. If you had no pins, the coin would go straight through at maximum speed
If you put a lot of pins, the coin will take a long time to arrive if at all.
The scientists in this study found an electrolyte that has almost no pins.
Now I understand that's not exactly what you meant...
What you asked is much simpler.
You can do a thought experiment and get the minimum order of magnitude of the differences. Let's forget for a moment about all the diffusion, objections and other kinds of vegetables.
We have an electron and a lithium ion (ie the charge of both is equal in absolute value), as in any standard rechargeable battery. The same potential, that is, the same force, acts on both. The ratio between the speeds of the electron and the lithium ion will be the root of the mass ratio:
m(1)*v(1)^2= m(2)*v(2)^2
The speed ratio I calculated in a rather rough calculation is around 110 times, that is, two orders of magnitude.
And in the ideal case.
Now it is true that the electron also has resistance to pass, but that is nothing compared to the resistance that an ion has. In terms of the size of both - the ion is much bigger.
Regarding the second question, it seems to me that it has a philosophical side among other things. There is no difference between an ion and an atom except for the charge, but it is worth emphasizing that this is about electric forces. I think it is worth noting that a moving particle has an electric charge.
Hope I helped 🙂
Roy
Ami
You left us pigeons without cargo..
interesting.
Greetings thirsty for confidential information :) in a puzzle..
=
Thanks for the explanation Roy. What settled everything for me in your explanation was the word "electrolyte" (although there is still a chance that I understood something wrong). They improved the electrolyte. It's very beautiful.
According to standard and famous calculations, the maximum speed of molecular oxygen in an aqueous medium at room temperature is of the order of 70 microns per second (this is what is called in Hebrew: diffusion). At high temperatures, of course, the speed is greater. But let's assume for a moment 70 microns per second. If they improved this speed, thanks to the electrolyte, by eight orders of magnitude, then in a complicated and unprecedented calculation I reach 70 ten minus 14 of a second (for the sake of the matter, let's assume that their improvement is from a micro second to a pico second).
My question is: what is the bottleneck in fuel cell reactions? What, other than the ion velocity, is the low velocity that follows the combustion/conduction/other process? Is a picosecond a lot or a little, let's say in relation to the speed of electron conduction in a gold wire at a temperature of one hundred degrees? Broadly speaking, what are the orders of magnitude to be reached?
The second and unrelated question, but semantically interesting: the difference between an ion and an atom whose outer shell of orbitals is not missing, is clear and known. The question is actually can an ion be called an atom? Of course, ion is opaque, but opaque with charge...
Oh, I had a phone call in the middle and my train of thought was interrupted and with it the motivation to write.
The second question can be waived. Maybe not on the first one.
With a thirst for information,
Ami Bachar
to my people,
Fuel cells work much like a battery, taking a neutral substance for example oxygen in fuel cells or lithium in batteries, and separating from it an electron instead of X. You move them both to one place with a lower chemical potential (otherwise they won't be "motivated" to move) and connect them to another compound.
The part where they break down into a positive ion (and not an atom) and an electron is known as the anode. The part where they come together is called the cathode.
Now, to transfer the electron you separated, you use a wire, where the current actually flows - the current is the electrons you transfer (and the voltage is the chemical potential difference between the anode and the cathode). You cannot transfer the positive ions through a cable, for them you need another medium, which is also known as an electrolyte, this is actually the medium they innovated. Because you can't just transfer an electron, but must transfer both, any improvement in ionic conduction will help you lower the overall resistance of the cell and actually make it more efficient.
what???,
In Hebrew, they managed to reach a good resolution, they will answer approximately they are able to see the sizes of individual atoms - the size of an atom is around an angstrom, and they can separate two particles whose distance between them exceeds 0.6 angstrom. Cool Dude?
"The characterization was done using a Z-contrast transmission-scanning electron microscope with a power of 300 kV, which is able to achieve a corrected resolution-defocusing of light rays close to 0.6 Angstroms, until recently - a world record. The direct images show the crystalline structure that explains the Conductivity of the material."
Is this paragraph written in Hebrew???
I learned from this article that pigeons need to go. Until now, I always thought that they were dealing with electron conduction, and now it's about the conduction of the atom. I have no idea what it's good for and how the physics of it work in fuel cells, but I guess "improvement" is a positive word and eight orders of magnitude is a lot.
Greetings friends,
Ami Bachar