Chemists from New York University have succeeded in making coiled coils - they fold into coiled spiral structures and will enable the development of medical materials and other chemicals inherent in complex structures
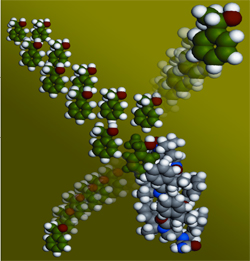
Chemists from New York University have succeeded in making coiled coils - they fold into coiled spiral structures - capable of accelerating selected chemical reactions. The research, the findings of which were published in the latest issue of the scientific journal Proceedings of the National Academy of Sciences (PNAS), could provide valuable methods for the preparation of medical materials and other chemicals inherent in complex structures.
The research team deals with the field called "biometic chemistry" (biometic - imitating nature). "This research was looking for synthetic compounds with structures and functions similar to those found in natural materials. Many biological materials, such as proteins and DNA, can fold into ordered coils and surfaces. In the last decade, scientists have succeeded in making molecular chains capable of folding into diverse structures. Although these materials, called "foldamers" (folding units, foldamers), resemble biochemical forms, finding those containing parallels in their biochemical functions has been much more elusive. Now, NYU chemists have succeeded in creating folded furrows capable of performing a complex function. In this case, the new products are used as catalysts - substances that speed up the rate of the chemical reaction itself without changing.
The article describes how a catalytic chemical group (catalyst) can be embedded within a larger coiled structure. The hypothesis of the researchers was that a controlled change of the coiled environment could help determine the degree of affinity between the catalytic group and the molecules in its environment. In order to test the activity of their materials, the researchers reacted them with particles that are mirror images of each other, that is, particles that have the same atomic composition but a different spatial arrangement of them, similar to left and right gloves, in order to determine if they will react properly with one of the pairs to obtain a chemical product New. This ability of the material was proof of the accuracy of its activity.
"Our molecules are particularly interesting because they are selective - they will recognize one unique type of target molecule and accelerate its chemical destruction," explains chemistry professor Kent Kirshenbaum, one of the authors of the paper. "This characteristic is particularly important in the preparation of complex chemical structures, so we believe that this could be useful, in the end, for the preparation of new drugs."
"Substances used in the pharmaceutical industry must be manufactured in a very specific and precise way," he adds. "The difference in the biological activity obtained between two mirror separations of this type (called enantiomers) may be enormous, so it is essential that the catalyst can correctly differentiate between very similar chemical structures. Once we understand the laws underlying the dependence between the various convoluted structures and the functions we desire, we will be able to lead our foals to perform many other and varied tasks."
An Israeli researcher, Dr. Galia Maayan, also participated in the research team.

2 תגובות
Blackmail my friend!!
Reminiscent of secondary or tertiary structure of proteins.