What is pressure in water and gas, how is pressure measured, and what is osmotic pressure?
Author: Zvi Atzmon, young Galileo
Pressure is a force exerted on a unit area. The greater the force, the greater the pressure. The pressure also increases as the area on which the force acts is smaller. For example, when you apply force to a pin whose tip touches the paper, the tip of the pin easily punctures the paper: the force acts on the very small area of the tip, and therefore the pressure is great and easily punctures the paper. If we apply the same force to a pin when its broad head touches the paper, a hole will not be created in the paper - the pressure will not be enough for that. In physics, pressure is denoted by the letter p.
What is water pressure?
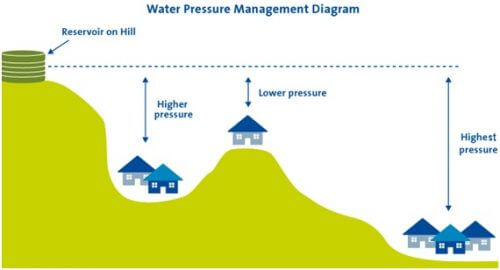
Inside water there is a pressure that the water creates - the deeper you go in the water, the higher the pressure. We feel it well when diving in a pool or in the sea and we have pressure in our ears. Not only water creates pressure, but any liquid. Pressure of a liquid column is called hydrostatic pressure. The hydrostatic pressure depends on the depth of the liquid and its specific gravity. The hydrostatic pressure in a liquid is the weight of a column of liquid per unit area. If you put a body in water to a depth of ten centimeters, pressure acts on it, but quite weak. A much stronger pressure acts on a body that is ten centimeters deep in mercury (a liquid with a high specific gravity). A water column of ten and a half meters creates a pressure of about one atmosphere (explained below), and a column of mercury 76 centimeters high also creates a pressure of one atmosphere. It is the pressure that increases with depth that limits the depth that submarines can go to without being damaged.
Does gas also create pressure?
Not only a column of liquid creates pressure but also a column of gas, but the specific gravity of gas is much lower than that of water. The air column from the surface of the sea to the upper parts of the atmosphere creates a pressure equal to the hydrostatic pressure of about ten and a half meters of water, or 76 centimeters of mercury. This pressure is called "one atmosphere".
Pressure can also be created by inflating - adding air to a limited space, like inflating air in a balloon, football, bicycle tire or car tire. As more air molecules are compressed into the tire, the pressure in it increases. There are metal cylinders that compress a large amount of carbon dioxide under high pressure to create soda.
How do you measure pressure?
Pressure is expressed in units of atmosphere (marked atm): at sea level the air pressure is about 1 atm. At a depth of ten and a half meters in the sea, the pressure is approximately 2 atm (the air pressure is 1 atm, and the pressure of the water column is 1 atm). On Everest, the air pressure is a little less than a third of an atm.
Pressure can also be expressed in units of the height of a column of liquid that creates such pressure. For example, atmospheric pressure is equal to a pressure of 760 millimeters of mercury. The pressure of one millimeter of mercury is called a torus - named after Torricelli, the scientist who proved that a vacuum (vacuum - zero pressure) can exist. Tires usually indicate the pressure in PSI units; 15 PSI equals about 1 atm.
What is osmotic pressure?
If you dissolve sugar or salt in water, you get a solution. It is customary to say that such a solution has an osmotic pressure. What is meant by? If we pour a sugar solution into a vessel whose sides are made of a material that allows water to pass through and prevents the passage of sugar molecules, and we put this vessel into a vessel containing pure water - water molecules will begin to pass through the sides into the sugar solution. This process is called osmosis. If you apply high enough pressure on the vessel that the sugar solution is in, water will stop penetrating the sugar solution. The higher the concentration of sugar in the sugar solution, the more pressure must be exerted on it to stop the osmotic entry of water into it.
Osmotic pressure is the pressure that must be exerted to prevent osmotic entry of water. If so, it is not pressure, but the need to apply pressure. Therefore it is more accurate to say that the solution has an osmotic value, and not an osmotic pressure. The units of the osmotic value of a solution are units of pressure (e.g. atmospheres), because that is the pressure that needs to be exerted on the solution. This value is denoted by the Greek letter Pi (π, equivalent to the English letter p, which, as you remember, indicates pressure). The cells of plants and bacteria have a high osmotic value - their cells are wrapped in a rigid wall that can exert pressure on them, thus preventing the osmotic entry of excess water and the danger of the cell exploding.
If we apply an external pressure on a solution greater than its osmotic pressure, not only will water not enter it by osmosis, but water will also leave it. This is reverse osmosis, and this method is used for water desalination - changing salty water into potable water (we wrote about reverse osmosis in Israel in the May 2016 issue).
In summary, pressure is force per unit area; In contrast, osmotic pressure is a pressure that must be applied to a solution to prevent water from entering it osmotically.
The article was published in the Galileo Young Monthly for curious children. For a gift digital sheet Click

One response
The article is about 50 years old... when I was a child they measured the atmospheric pressure. But the atmosphere does not have an exact basic unit of size (and I will explain why shortly). Also, it does not fit with the unit system currently accepted in physics, chemistry and engineering (the MKSA method, meter kg-second and amperes), so the poor atmosphere was replaced by the "Pascal" unit, and since the Pascal is a very small measure of pressure in engineering, it is replaced by a unit derived from it, which is the "bar" ". One bar is equal to one hundred thousand pascals and this is a fundamental measure and an apartment! And why exactly a bar and the answer is that 1 atmosphere is roughly equal to 1 bar and here is the explanation for the beginning, the atmospheric pressure fluctuates every day between 1010 and 1033 millibars (depending on the day) and therefore it is not accurate and rare and in general in recent years the students are already taught to get used to going back to the basic course and use MPA units That is, megapascal or 10 sixths of a pascal, which is exactly a pressure 10 times greater than a bar.